Carlos Eduardo R. Velos; Márcio B. Nehemy
DOI: 10.17545/eOftalmo/2023.0010
ABSTRACT
Polypoidal choroidal vasculopathy is a subtype of age-related macular degeneration whose treatment usually involves the use of anti-vascular endothelial growth factor agents, which may be associated with photodynamic therapy. The current case report demonstrates the use of brolucizumab in a patient with polypoidal choroidal vasculopathy resistant to other anti-vascular endothelial growth factor agents, providing excellent anatomical and functional responses.
Keywords: Exudative macular degeneration; Vascular endothelial growth factor; Optical coherence tomography.
RESUMO
A vasculopatia polipoidal da coroide é um subtipo da degeneração macular relacionada à idade cujo tratamento geralmente envolve o uso de agentes anti-VEGF associados ou não à terapia fotodinâmica. O presente caso demonstra o uso do brolucizumabe em um caso de vasculopatia polipoidal da coroide resistente a outros agentes anti-VEGF proporcionando excelente resposta anatômica e funcional.
Palavras-chave: Degeneração macular exsudativa; Fator de crescimento do endotélio vascular; Tomografia de coerência óptica.
INTRODUCTION
Polypoidal choroidal vasculopathy (PCV) is a subtype of neovascular age-related macular degeneration (AMD) characterized by type 1 choroidal neovascularization associated with a branching vascular network with aneurysmal dilations1. Indocyanine green angiography (ICGA) remains the gold standard diagnostic method, but optical coherence tomography (OCT) is a more widely available propaedeutic method, revealing important findings such as inverted U-shaped retinal pigment epithelial detachment (PED)2,3. PCV, which is known to be common in Asians, has a high prevalence in Brazil. A study conducted in Brazilian patients with neovascular AMD revealed that 24.5% of patients had PCV4. The current treatment of PCV involves the use of anti-vascular endothelial growth factor (VEGF) agents, which may be associated with photodynamic therapy (PDT). Recurrent subretinal hemorrhages and persistent fluid accumulation (resistant to anti-VEGF) are the complications of this disease, which, if not treated adequately, can lead to permanent visual loss5,6.
Brolucizumab is a new molecule recently approved for use in Brazil and is considered as the smallest functional unit of an antibody with receptor binding ability to VEGF. Its low molecular weight maximizes the amount of anti-VEGF agent that can be injected into the vitreous cavity7. The randomized HAWK/HARRIER clinical trial evaluating the efficacy and safety of this drug showed that compared with aflibercept, approximately 50% of patients can be treated with brolucizumab with a longer interval between applications, providing excellent functional and better anatomical responses8. A recent subanalysis of this study in Japanese patients with PCV also showed favorable results for brolucizumab compared with aflibercept9, with higher resolution of subretinal and sub-RPE fluid.
This clinical case report illustrates the use of brolucizumab in a patient with PCV resistant to other anti-VEGF agents, providing both anatomical and functional improvement.
CLINICAL CASE
A 74-year-old woman with pseudophakia in both eyes complained of worsening of visual acuity of the left eye (OS), which started in January 2021. The visual acuity of the right eye (OD) was 20/20, whereas that of the left eye (OS) was 20/60.
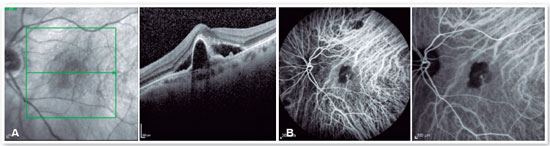
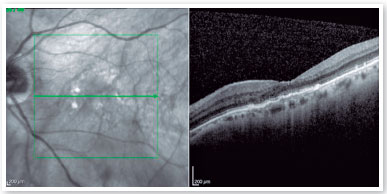
OCT of the left eye revealed the presence of subretinal fluid, inverted U-shaped retinal pigment epithelial detachment (PED), subretinal hyperreflective material, and double layer sign (Figure 1A). The central choroidal thickness was subnormal (174 µm), but the presence of choroidal pachyvessels was noted in the area underlying the PED. Considering these tomographic findings suggestive of PCV, ICGA was indicated, which revealed the presence of a hypercyanescent lesion within the first 6 min of examination, corresponding to the polyp area identified in OCT, in addition to a surrounding hypocyanescent area (Figure 1B). Color fundus photography and fluorescein angiography complemented the initial propaedeutics. Scans of the right eye revealed no changes.
The patient was then subjected to a loading dose of three monthly intravitreal injections of aflibercept in the left eye. After this treatment, visual acuity worsened to 20/150 and the tomographic findings remained unchanged (Figure 2A). A fourth intravitreal injection of aflibercept was indicated; however, no tomographic or functional improvement was noted (Figure 2B).
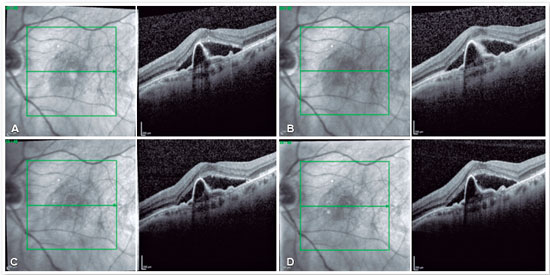
At that time, as PDT was impossible due to the shortage of verteporfin in the country, a fifth intravitreal injection of the anti-VEGF agent was indicated along with ranibizumab. Once again, no anatomical or functional improvement was noted (Figure 2C). A sixth intravitreal injection of an anti-VEGF agent (aflibercept) was subsequently administered, revealing the same anatomical and functional responses (Figure 2D).
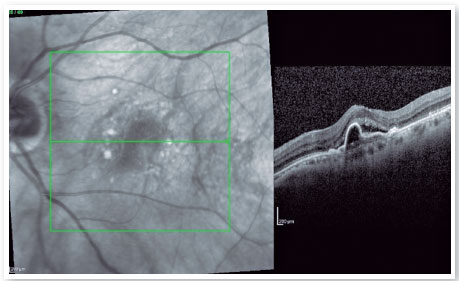
A seventh intravitreal injection of an anti-VEGF agent (ranibizumab) along with intravitreal injection of triamcinolone (4 mg/mL) was then administered. This combined treatment showed a partial tomographic improvement but did not indicate any functional improvement (Figure 3).
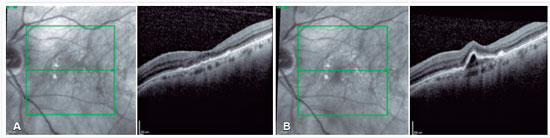
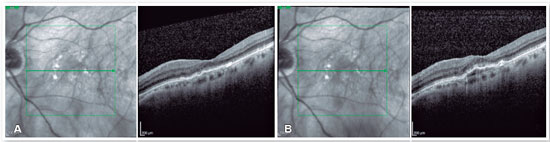
At that time, brolucizumab was approved for use in Brazil; accordingly, the drug was indicated for use in this patient. One month after intravitreal injection of brolucizumab, her visual acuity improved to 20/100 and showed excellent anatomical response, with complete resolution of PED and subretinal fluid (Figure 4A). OCT was performed 8 weeks after intravitreal injection of brolucizumab, which revealed PED growth without associated intra- or subretinal fluid (Figure 4B). We then administered a new intravitreal injection of brolucizumab, which provided excellent anatomical and functional responses (Figure 5A). Eight weeks after the second dose of brolucizumab, a new PED growth was observed, but without any associated intra- or subretinal fluid (Figure 5B). The third intravitreal injection of brolucizumab was subsequently administered. OCT performed 4 weeks after the third dose revealed complete fluid resolution (Figure 6). The patient is currently under follow-up.
DISCUSSION
The current case presents angiographic and tomographic characteristics suggestive of PCV. In ICGA, the hyperfluorescent lesion observed within the first 6 min of the examination is considered as one of the main diagnostic criteria of PCV5. The presence of inverted U-shaped PED, double layer sign, and multilobulated PED are among the tomographic signs suggestive of PCV2,5. The elevated feature of inverted U-shaped PED is considered the most frequent tomographic sign in patients with PCV. A recent study evaluating 69 eyes with PCV revealed that this characteristic was observed in 56.4% of the cases2. Another tomographic finding noted in this case was reduced choroidal thickness (175 μm) and the presence of pachyvessels in the area underlying the PED. In a study involving 320 eyes with PCV, Lee et al.3 revealed a bimodal distribution of subfoveal choroidal thickness, with peaks of 170 and 390 microns. Moreover, in the group with reduced choroidal thickness, pachyvessels were noted in the area underlying the polypoidal lesion, similar to the current case.
In our case, resistance to treatment with various intravitreal injections of aflibercept and ranibizumab was observed. It is believed that patients with PCV have a higher resistance to treatment with these drugs than those with the classic form of AMD. Kokame et al.6 evaluated 253 eyes with neovascular AMD (114 eyes with PCV) and found that PCV was diagnosed in 50% of the eyes that were resistant to anti-VEGF therapy (persistent subretinal fluid, macular edema, or subretinal hemorrhage after four sequential intravitreal injections). In the current case, we tried a combined treatment with intravitreal anti-VEGF and corticosteroids but there was only a partial anatomical improvement. Although corticosteroids are an off-label drug, they can be exceptionally used in cases of nonresponsive neovascular AMD10.
Brolucizumab was the only drug used in this case that provided excellent anatomical and functional responses. Rapid and complete resolution of subretinal fluid was observed immediately after the first dose of brolucizumab, which may represent the inactivity (at least temporary) of the polyp. Notably, although the drug was used in this case every 8 weeks, after this treatment, no intra- or subretinal fluid was noted and only one PED growth was observed. The indication for anti-VEGF therapy remains controversial in a patient undergoing treatment for neovascular AMD with PED growth and the absence of intra- or subretinal fluid. Some studies have indicated treatment, whereas others have suggested using a more conservative approach11.
A recent subanalysis of the HAWK study conducted in Japanese patients with PCV revealed that brolucizumab (6 mg) monotherapy (every 8 or 12 weeks) resulted in robust and consistent visual improvements, comparable to those obtained with aflibercept monotherapy every 8 weeks. However, the anatomical results obtained with brolucizumab were clearly superior compared with aflibercept. At the end of 48 weeks of treatment, intra- or subretinal fluid was noted in 7.7% of patients treated with brolucizumab and 30% of those treated with aflibercept. At the end of 96 weeks, intra- or subretinal fluid was noted in 12.8% of patients treated with brolucizumab and 16.7% of those treated with aflibercept. Similarly, a lower proportion of eyes with sub-RPE fluid was observed in patients treated with brolucizumab than in those treated with aflibercept from week 4 (38.5% vs. 60.0%) to 96 (7.7% vs. 13.3%), respectively. In addition, most of the studied patients (76% at the end of 48 weeks and 68% at the end of 96 weeks) received this drug every 12 weeks9.
As brolucizumab monotherapy results in fluid resolution in a higher percentage of patients, it may represent an alternative treatment option for patients with PCV who would have traditionally been administered a combined treatment (anti-VEGF and PDT). Thus, published literature and the results obtained in the current case indicate that brolucizumab can be considered particularly important in the treatment of PCV, providing excellent anatomical (superior to aflibercept) and functional responses, thereby allowing a greater interval between the applications.
REFERENCES
1. Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127(5):616-36.
2. Kokame GT, Omizo JN, Kokame KA, Yamane ML. Differentiating exudative macular degeneration and polypoidal choroidal vasculopathy using OCT B-scan. Ophthalmol Retina. 2021;5(10): 954-961.
3. Lee WK, Baek J, Dansingani KK, Lee JH, Freund B. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016;36 Suppl 1:S73-S82.
4. Pereira FB, Veloso CE, Kokame GT, Nehemy MB. Characteristics of neovascular age-related macular degeneration in brazilian patients. Ophthalmologica. 2015;234(4):233-42.
5. Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, Freund KB et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125(5):708-24.
6. Kokame GT, DeCarlo TA, Kaneko KN, Omizo JN. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744-52.
7. Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127(7):963-76.
8. Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG; HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127(1):72-84.
9. Ogura Y, Jaffe GJ, Cheung CMG, Kokame GT, Iida T, Takahashi K, et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2022;106(7):994-9.
10. Kaya C, Zandi S, Pfister IB, Gerhardt C, Garweg JG. Adding a corticosteroid or switching to another anti-VEGF in insufficiently responsive wet age-related macular degeneration. Clin Ophthalmol. 2019 Dec 5;13:2403-2409.
11. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016 Jan;50:1-24.

Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
November 30, 2022.
Accepted on:
December 18, 2022.