Sidney J Faria-e-Sousa1; Stella Barretto2
DOI: 10.17545/eoftalmo/2018.0003
ABSTRACT
This article focus on in vitro analysis of the endothelium of donated corneas for transplantation. It is about the influence of preservative solutions on the endothelium shape, the morphological and morphometric analyses of the endothelial layer, the cellular pattern of acute endothelial losses and its differential diagnosis, the role of endothelial morphology on the regulation of corneal hydration, and specular microscopy.
Keywords: Endothelium; Eye Banks; Corneal Transplantation.
RESUMO
Este artigo concentra-se na análise in vitro do endotélio das córneas doadas para transplante. Ele se refere à influência das soluções preservativas sobre o aspecto do endotélio, a lógica das análises morfológicas e morfométricas do mesmo, o padrão das perdas endoteliais agudas e seu diagnóstico diferencial, a influência da morfologia endotelial sobre regulação da hidratação da corneana e a microscopia especular.
Palavras-chave: Endotélio; Banco de Olhos; Transplante de Córnea.
RESUMEN
Este artículo presenta el análisis in vitro del endotelio de las córneas donadas para trasplante. Su contenido se refiere a la influencia de las soluciones preservativas sobre el aspecto del endotelio, la lógica de sus análisis morfológicos y morfométricos, el estándar de las pérdidas endoteliales agudas y su diagnóstico diferencial, la influencia de la morfología endotelial sobre la regulación de la hidratación de la córnea y la microscopía especular.
Palabras-clave: Endotelio; Banco de Ojos; Trasplante de Cornea.
INTRODUCTION
Corneal selection for transplantation comprises four stages: ocular inspection in the dead body, in situ corneal analysis, in vitro corneal analysis, and endothelial specular microscopy. Inspection involves observing the donor’s eyes, eyelids, and orbits to look for signs of trauma, jaundice, bleeding, purulent secretion, conjunctival tumors, and foreign bodies. Inspection is performed with the naked eye and with the assistance of a pocket flashlight. In situ analysis is performed on a slit lamp with the cornea seated in the eyeball1. It aims to detect abnormalities that might interfere with the transparency and safety of the graft by evaluating the epithelium, stroma, endothelium, and anterior chamber. In vitro analysis is the examination of the cornea within the preservation medium. The main advantage of this procedure is to allow the observation of the endothelium from the inner side of the cornea without interference of the swollen stroma. It is performed with a slit lamp through a preservation bottle. The most sophisticated preservation agents are marketed in high-transparency plastic chambers that are specially designed for biomicroscopic examination. Eye banks that work with glass vials benefit from the Cowden analyzer2. This instrument holds the storage bottle upright, and through a 45° tilted mirror, it projects its bottom, along with the corneoscleral button, in the horizontal plane. With a magnification of 20 to 30 times, the details of the endothelial mosaic may be seen (Figure 1).

Once the cornea has been concluded to have satisfactory transparency and to pose no obvious risk to the host, the focus changes to the study of its vitality, i.e., its ability to remain transparent in the recipient’s eye. This feature depends on the endothelial functional reserve, which actively controls the graft hydration status and, consequently, its transparency. Unfortunately, no non-invasive method of evaluating this parameter currently exists3, 4. Cell counting per unit area (endothelial density) and morphological and morphometric analyses of the endothelial mosaic are allegedly the best indirect information available to infer about the health and function of endothelial cells. The logic behind these procedures is the supposition that changes in number, shape, and size of cells directly affect endothelial performance, and vice versa. The extent to which this assumption is valid is unknown. Despite these limitations, the objective of in vitro analysis is to select corneas for grafting with the best chances of survival based on the characteristics of the endothelial mosaic.
INFLUENCE OF PRESERVATION ON THE ENDOTHELIAL SHAPE
In the postmortem period, as oxygen falls below a critical level, the cornea starts using anaerobic glycolysis, the process in which glucose breaks down into lactate, which accumulates in the stroma in the form of sodium lactate. This salt generates an osmotic effect that attracts fluid into the stroma. With prolonged hypoxia, the depletion of the glycogen stores and the decrease in the amount of adenosine triphosphate (ATP) lead to the deceleration of the water transport from the stroma to the anterior chamber. The combined effect of these events results in corneal edema5. The cornea might acquire up to twice the standard thickness when stored in a liquid medium. This occurrence is an inconvenience to corneal surgeons because very thick corneas are an obstacle to proper suture placement. To minimize edema, hyperosmotic agents such as dextran and chondroitin sulfate were added to liquid media. However, it was soon recognized that they could cause cell intoxication when ingested by the endothelial cells6-9. This lead to their withdrawal from organ culture media, because temperatures above 30ºC enhance cell phagocytosis. Nevertheless, they were maintained in cold preservation media because the hypothermia of 4ºC preservation reduces intracellular penetration10.
In cold preservation media, the endothelial mosaic is exposed to two opposing tendencies: edema, due to hypothermic inhibition of the endothelial pump, and dehydration, which is promoted by hyperosmotic agents. In edema, endothelial cells become abnormally bulging; in dehydration, endothelial cells become abnormally thinned with a protruding nucleus. In both cases, cell limits become poorly defined, thereby hampering endothelial analyses and counting. Experience shows that with the elevation of tissue temperature, endothelial cells recover regular hydration and cellular boundaries. Thus, keeping media storage bottles for at least 40 minutes at room temperature (23°C) before tissue analyses is advisable. However, a controlled study indicated that the optimal time for full visibility of the endothelial mosaic is 2 hours at least11. A high visibility of the mosaic corresponds to an improved chance of correct estimation of endothelial density (cells/mm2).
LOGIC OF MORPHOLOGICAL ANALYSES OF THE ENDOTHELIUM
The logic of morphological analyses of the endothelium relies on the following observations: from 5 years of age until death, a loss of approximately 0.6% endothelial cells per year occurs12. The cell decline accelerates with intraocular surgeries, anterior chamber inflammation, corneal anoxia, glaucoma, and guttata dystrophy13-17. As a result of the absence of endothelium reproduction, dead cells leave vacant spaces to be filled by adjacent cells that extend or slide toward the lesion. Abiding by this rule, endothelial layer acquires a complex visual aspect with a variety of cell shapes and sizes18,19,20.
In the short term, high endothelial loss corresponds to great cell variability21, 22. In the long run, the hexagonal pattern tends to recover 20, with the inner surface of the cornea becoming lined by a uniformly shaped endothelium with enlarged cells (Figure 2). The experienced examiner is capable of making a rough estimate of cell density based only on cell size; a large cell size indicates a small number of cells.
ACUTE ENDOTHELIAL LOSSES
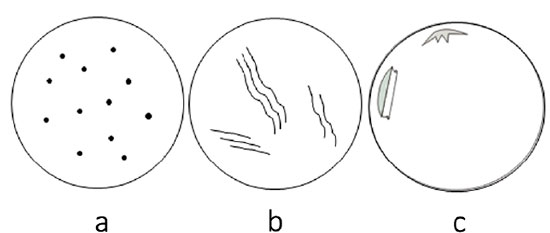
Acute endothelial losses due to mechanical injuries to the cornea before death leave empty spaces in the endothelial mosaic, justified by the absence of time for compensatory response. These voids appear as dark or grayish spots distributed in two basic patterns: focal and linear (Figure 3)23. They are spread randomly along the endothelial mosaic. Sinuous linear-shaped cell losses are referred to as “snail tracks”24.
The pattern of cell loss suggests the nature of the trauma. For example, focal losses scattered throughout the endothelial mosaic similar to birdshots indicate compressive frontal corneal trauma (Figure 4a). Snail tracks (Figure 4b) mean that the injury was either due to excessive corneal pull during corneal button removal or secondary to cell squeezing in the recesses of Descemet’s folds. Detachments of Descemet’s membrane suggest inadvertent endothelial injury by surgical instruments during corneal excision (Figure 4c).
CORNEA GUTTATA
Endothelial cells secrete collagen, forming Descemet’s membrane. During intrauterine life, this structure presents a banded configuration, reaching a thickness of approximately 3 μm at birth. Throughout life, it continues to grow in thickness, but with a non-banded homogeneous collagen. At 70 years old, its total thickness is around 13 μm25. When inflammation, trauma, or genetic disorders irritate endothelial cells, they may respond by producing fetal collagen at an accelerated rate 26,27, leading to either a diffuse or a focal reactive thickening of Descemet’s membrane. Focal lesions protrude to the anterior chamber in a verrucous pattern. The average cornea usually presents a small number of these nodular lesions in the periphery under the name of Hassall-Henle corpuscles; they have no clinical importance. However, when they concentrate in the central cornea in an ever-growing number with a gradual spreading to the periphery, one must consider the presence of a progressive disease called cornea guttata or guttata dystrophy. The typical lesion is the “gutta,” which means “drop.” Guttata describes the dewy aspect of the affected endothelium. Despite having an extruded profile, the guttae generate the illusion of dark holes under specular reflection (Figure 5c). In the beginning, the guttae are smaller than a cell. As the disease progresses, they acquire the size of several cellular areas. Endothelial cells covering the lesions become increasingly broader and thinner.
Cornea guttata is an intrinsic illness of the endothelial cell 28, which affects both eyes asymmetrically. It usually manifests after the age of 50 and is more prevalent in women29. The dystrophic disease induces cells to produce an excess of fetal basement membrane and, in that process, drastically shortens their life. The corneal stroma swells when the number of cells becomes insufficient to maintain the fluid balance of the cornea. Cornea guttata plus stromal edema configures a condition called Fuchs’ dystrophy. Vision declines with increasing swelling; above a certain level of corneal edema, the epithelium becomes involved, showing bullae that cause intermittent pain and the sensation of a foreign body when ruptured. Focal or diffuse thickening of Descemet’s membrane resulting from infection, inflammation, or trauma configures a secondary guttata. In this variety of guttata, lesions confine themselves to the area of the primarily affected endothelium, a detail that helps rule out the real dystrophy.
Some eye banks distribute corneas with an ordinal quantification of the guttata. In this way, they share with surgeons the responsibility of appreciating the significance of their findings. In our view, this practice is unfair; usually, surgeons do not have enough experience to decide on the subtleties of the in vitro examination. If the eye bank has no doubts about the innocence of the lesions found in the corneal endothelium, then it should distribute the tissue without further considerations. Otherwise, the cornea must be delivered only to transplantations where no endothelial exchange is required.
DIFFERENTIAL DIAGNOSIS BETWEEN ENDOTHELIAL LESIONS
Endothelial losses (Figure 5a) and guttata lesions (Figure 5c) manifest as dark spots in the endothelial mosaic during in vitro analysis. Differential diagnosis between these two entities is relevant. In the former, the cornea may be used, provided the endothelial density and morphology are within acceptable limits. In the latter, the cornea might be harboring a disease that disqualifies it for conventional transplants, regardless of the endothelial counting. To further complicate matters, a third possibility also has to be considered: endothelial vesicles or pseudoguttatas. They also manifest as dark spots in the endothelial mosaic (Figure 5b). They are linear agglomerates of swelled endothelial cells, similar to those observed on wearers of contact lenses with low oxygen permeability21. Apparently, these lesions result from the combined effect of the increased permeability of the endothelial cell membrane, secondary to postmortem hypoxia 30, and tissue hypothermia associated with 4ºC preservation. They disappear with the elevation of the storage medium temperature. This event is another reason to keep medium storage bottles at room temperature (23 °C) for at least 40 minutes before analyzing the tissues.
Sometimes, differential diagnosis between guttata and cell loss becomes challenging. In this situation, useful details to remember are that cornea guttata affects both eyes, that guttae are always more numerous in the center of the cornea, and that the disease is more prevalent in women above 50 years old. Endothelial losses show a random distribution without association with sex or age. In many cases, specular microscopy removes the grounds of severe doubts due to its higher magnification.
ENDOTHELIAL MORPHOLOGY AND REGULATION OF CORNEAL HYDRATION
The endothelium controls the hydration of the cornea to keep it slim and clear. The prevailing hypothesis is that a fluid exchange between the cornea and the anterior chamber described as a “pump–leak” arrangement fulfills this task. Accordingly, specialized endothelial membrane proteins pump the fluid absorbed by the stoma back to the anterior chamber. Even though its operational details are not fully understood, this process seems to require Na+, K+, HCO3 -, Cl-, ATPase, and carbonic anhydrase to function. Apparently, the process relies on the anion transport system of the basolateral wall (facing the stroma) and in water and ion transport channels of the apical wall (facing the aqueous humor) of endothelial cells31.
The force with which the cornea absorbs water is called imbibition pressure, which stems from the hygroscopic properties of the glycosaminoglycans scattered among stromal lamellae32. The passive flow of fluid through the endothelial layer from the anterior chamber to the corneal stroma uses the paracellular pathway (the interval between cells), which is regulated by the tight junctions at the apical membrane of the cells33. The stroma swells when pumping fails to compensate for leakage; imbibition pressure decreases with edema progression, and this cycle stops only with the restoration of the fluid balance under a high level of corneal thickness.
Paracellular endothelial leakage stirred interest in the influence of the intercellular space on corneal hydration. Endothelial layer is formed by regular hexagonal cells. Among the three regular patterns of tessellation — triangle, square, and hexagon — the last is the one that has the shortest perimeter per unit area. Consequently, the length of the intercellular space of the endothelial mosaic is the smallest achievable length. This anatomical detail probably spares pump work by leaving minimum room for fluid leakage. Throughout life, the natural loss of endothelial cells generates a mosaic with ever larger cells. As long as it preserves uniform cell configuration, large cells correspond to a small intercellular space facing the anterior chamber. An increase of x times in the cell area generates an increase of √x times in the perimeter of the intercellular space. In relative terms, this condition corresponds to a decrease in the intercellular space. This property is advantageous to the cornea because it decreases the pumping demand as the cell density decreases. Studies in humans and animals confirmed that big cell sizes associate with a low permeability of endothelial mosaic34-36. Conversely, as the variability of the cell sizes increases, the extension of the intercellular space enlarges, leading to more leakage through the paracellular route. These observations support the assumption that endothelial mosaics with uniform cell size and shape are the ones that best maintain the barrier function37.
Bourne et al. formulated the theory that a decrease in the functional reserve of the endothelium could be explained exclusively by the reduction of the extension of intercellular space34,36,38. One of the premises of this hypothesis is that the enzymes responsible for the pump function concentrate exclusively on the lateral walls of the endothelial cells; with the diminution of the intercellular perimeter, these enzymes would become more scarce39,40. Later studies, however, showed that pump enzymes pervade the entire cellular membrane31. Also, O’Neal and Polse found a significant decrease in the pump function in the irregular endothelial mosaic41, which has an augmented intercellular extension due to geometrical reasons. Polse et al.42 proposed that age, by itself, could be a factor in the diminished control of corneal hydration. This proposal was crucial in suggesting that other factors besides cell geometry could influence the regulation of corneal hydration. Currently, studies on this subject have focused on the physiology of the pump itself and the mechanisms of paracellular flow control31,33.
Irrespective of the controversies about the control of corneal hydration, an important information emerges from this matter. With aging, the endothelium loses cells, thereby leading to either a homogeneous mosaic of large cells or an irregular mosaic with high variability of cell sizes and shapes. Despite the first scenario being conducive to less leakage, both present a decrease in the control of corneal hydration for reasons not yet clarified36,41. This observation supports the conjecture, derived from common sense, that healthy old corneas should not perform the same as healthy young corneas.
POLYMEGETHISM AND PLEOMORPHISM
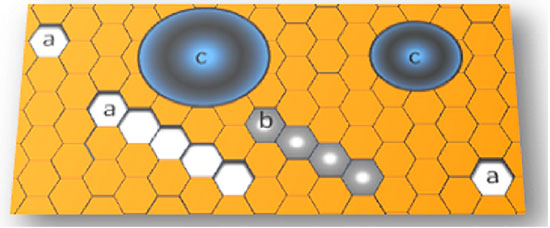
Polymegethism is the excessive variability of the cell area, evidenced by an endothelial mosaic composed of cells of mixed sizes43. The phenomenon is quantified by the coefficient of variation of the endothelial cell area (CV), i.e., the quotient of the standard deviation and mean area of cells from a specific field of observation. The expected value is at most 30% in the healthy adult endothelium5,22. Another way of quantifying polymegethism is by the ratio between the maximum and minimum cell size, which should be less than 522. Pleomorphism is the excessive variability of the cell shape. As it increases, the percentage of hexagonal cells decreases. In the healthy endothelium, at least 60% of the cells of a specific field should be hexagonal44. Polymegethism and pleomorphism are interconnected by Mullins–von Neumann’s law45, which states that areas with more than six sides expand and those with fewer sides shrink, with the balance represented by the hexagonal configuration (Figure 6).
Despite our tendency to associate polymegethism with the healing process of endothelial cells, polymegethism may also arise from tissue hypoxia, unrelated to cellular losses22,46, as observed in wearers of contact lenses with low permeability to oxygen47-49. Variability of cell area without a corresponding change in endothelial density (cells/ mm2) is justified by the enlargement of some cells at the expense of the reduction of others50. Another attractive possibility is that the size of cells do not change; only a spatial reorientation of the cellular mass exists so that cells of small basal area show large apical surfaces, and vice versa51.
An intriguing event is the concomitance of polymegethism with normal endothelial density in non-contact lens wearers37,52. This finding calls for a third source of polymegethism unrelated to either cell loss or hypoxia. Assuming that cell size variability increases with age, this third source may be a combination of factors in which cell aging might participate53.
The functional reserve is the capability of the endothelial layer to revert corneal edema52. Corneas with the same endothelial density may show distinct levels of recuperation from the edema caused by hypoxia or trauma37,52,54,55,56. This observation brought about the concept that the estimation of cell density is an important practice but is not sufficient for anticipating the functional reserve of the endothelium. In the search for other diagnostic parameters, interest turned to the morphological variations of the endothelial mosaic and, more specifically, to polymegethism and pleomorphism52. Several studies found that polymegethism correlates with a lower ability to reverse corneal edema derived from intraocular surgeries or contact lens wear37,43,54,55,56. As a consequence, polymegethism has been interpreted as a manifestation of endothelial stress that precedes or49 succeeds cell loss. Under this understanding, corneas with abnormal CV would have lower chances of survival after corneal transplantation. This reasoning would be robust if polymegethism would derive exclusively from cell loss. But its coexistence with no such event opens the possibility of mosaic recovering into preservative medium or host eye environment. In the literature, remarks have already been made on this subject57,58. This kind of doubts on the interpretation of the changes of the endothelial layer morphology is one of the reasons endothelial density is still the most objective criterion for tissue selection so far.
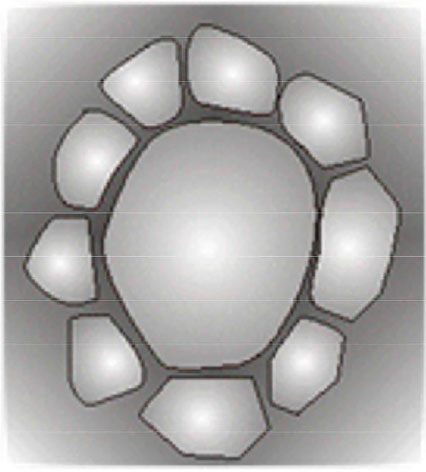
Modern specular microscopes employ computational programs to quantify polymegethism and pleomorphism. Although numerical limits of normality for these phenomena have already been established, no consensual protocol for corneal selection based on this information is available so far. This void is another reason endothelial density is still the dominant parameter for the endothelial selection of donated corneas. In the absence of automatic endothelial cell counting, the search for rosettes — cell formations where several small cells surround a larger one — may be helpful as their frequency increases with the exacerbation of polymegethism (Figure 7)37,46. However, this procedure has the same limitation of computational analysis, i.e., the lack of a definition about the number of rosettes per field of view that will render the cornea unsuitable for transplantation.
SPECULAR MICROSCOPY
Specular microscopy is the next exam after in situ analysis of the corneal endothelium and is part of the in situ analysis sensu lato. The instrument projects a light beam into the cornea and captures the reflected image of the interface between the endothelium and the aqueous humor. The advantages of the specular microscope over the slit lamp are larger images and automated cell counting. Although cell density is not the only parameter in the assessment of endothelium quality, it is still the most concrete information available to eye bank professionals. When using endothelial density as a selection criterion, the assumption is that a big cell count corresponds to a high probability of graft survival. In fact, numerous publications support this interpretation when considering the initial endothelial density as the chief influential factor in graft survival59-61.
As both duration of permanence in cold storage medium62-64 and surgery tend to decrease the endothelial graft density, eye banks take those factors into account when defining the minimum cell density for a donor cornea to be eligible for grafting. In a sample of 72 clear corneal grafts with 10 years, Ing et al65. observed that they had approximately one-third of the initial cell density with a mean cell area of 958 ± 471 mm2. Many eye banks, including ours, arbitrarily set the minimum endothelial density of 2,000 cells/ mm2 to qualify a donor cornea for grafting66. On the basis of the above values, the mean endothelial density expectancy at the end of 10 years is 660 cells/mm2. This number is still compatible with the transparency of the grafts67.
REFERENCES
1. Transplantation of the cornea. Arch Ophthamol.1935;13(3):321-347. https://doi.org/10.1001/archopht.1935.00840030011001
2. Cowden JW. Slit-lamp attachment for examination of donor corneas in MacCarey-Kaufman medium. Arch Ophthalmol.1979;97(5):953. https://doi.org/10.1001/archopht.1979.01020010511029
3. Horn DLV, Schultz RO. Corneal preservation: Recent advances. SurvOphthalmol 1977;12(4);301-312. https://doi.org/10.1016/0039-6257(77)90112-6
4. Wilson SE, Bourne WM. Corneal preservation. SurvOphthalmol 1989;33(4):237-259. https://doi.org/10.1016/0039-6257(82)90150-3
5. Liesegang TJ. Physiologic changes of the cornea with contact lens wear.The CLAO Journal 2000;28(1):12–27.
6. Lindstrom RL, Doughman DJ, Skelnik DL, Mindrup EA. Corneal preservation at 4C with chondroitin sulfate containing medium. Trans Am OphthalmolSoc 1987;85,1987.
7. McCarey BE, Kaufman HE. Improved corneal storage. Invest Ophthalmol Vis Sci1974;13:165-173.
8. Lindstrom RL, Kaufman HE, Skelnik BS, et al. Optisol corneal storage medium. Am J Ophthalmol 1992;114(3):345-356. https://doi.org/10.1016/S0002-9394(14)71803-3
9. Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. IntOphthalmol. 2008 Jun; 28(3): 155–163. doi: 10.1007/ s1079200790861
10. Faria-e-Sousa SJ, Barretto S. Preservação de córneas: uma breve história. Corneal preservation: A brief history. E-Oftalmo.CBO. Submitted to publication.
11. Pham C, Hellier E, Vo M, Szczotka-Flynn L, Benetz BA, Jonathan H. Lass J H. Donor Endothelial Specular Image Quality in Optisol GS and Life4ºC International Journal of Eye Banking 2013;1(2):1-8.
12. Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial changes over a ten-year period. Invest Ophthalmol Vis Sci 1997;38(3):779-782.
13. Bourne WM, Kaufman HE. Endothelial damage associated with intraocular lenses. Am J Ophthalmol 1976;81(4);482-486. https://doi.org/10.1016/0002-9394(76)90305-6
14. AlfawazAM, Holland GM, Yu F et al. Corneal endothelium in patients with anterior uveitis. Ophthalmology 2016; 123(8):1637-1645 https://doi.org/10.1016/j.ophtha.2016.04.036.
15. McMahon TT, Polse KA, McNamara N, et al. Recovery from induced corneal edema and endothelial morphology after long-term PMMA contact lens wear. Optom Vis Sci 1996;73:184–188. https://doi.org/10.1097/00006324-199603000-00010
16. Spencer WH, Ferguson WJ Jr, Shaffer RN, Fine M. Late degenerative changes in the cornea following breaks in the Descemet's membrane. Tran Am AcadOphthalmolOtolaryngol 1966;70(6):973-983.
17. Elhalis H, Azizi B, Jurkunas UV. Fuchs Endothelial Corneal Dystrophy. Ocul Surf. 2010;8(4):173–184. https://doi.org/10.1016/S1542-0124(12)70232-X
18. DoughmanDJ, Van Horn D, Rodman WP, Byrnes P, Lindstrom RL. Human corneal endothelial layer repair during organ culture. Arch Ophthalmol. 1976; 94(10):1791-1796. https://doi.org/10.1001/archopht.1976.03910040565016
19. Matsuda M, Sawa M, Edelhauser HF, Barrels SP, Neufeld AH, Kenyon KR. Cellular migration and morphology in corneal endothelial wound repair. Invest Ophthalmol Vis Sci. 1985; 26 (4):443-449.
20. Yee RW, Geroski DH, Matsuda M, Champeau EJ, Meyer LA, Edelhauser HF. Correlation of corneal endothelial pump site density, barrier function, and morphology in wound repair. Invest Ophthalmol Vis Sci. 1985; 26 (9):1191-1201.
21. ZantosSG and Holden BA: Transient endothelial cell changes soon after wearing soft contact lenses. Am J OptomPhysiolOpt 1977;54(12):856-858. https://doi.org/10.1097/00006324-197712000-00010
22. Stocker EG, Schoessler JP. Corneal endothelium polimegatism induced by PMMA contact lens wear. Invest Ophthalmol Vis Sci 1985;26(6):857-863.
23. Marcomini LAC, Sobral RMCR, Seixas GO, Faria e Sousa SJ. Seleção de córneas para transplantes. Rev. Bras. Oftalmol 2011;70(6):430-436. https://doi.org/10.1590/S0034-72802011000600020
24. Alfonso E, Tucker GS, Battle JF, Mandelbaum S, Gelender H, Forster RK. Snailtracks of the corneal endothelium. Ophthalmology 1986;83(3):344-349 https://doi.org/10.1016/S0161-6420(86)33736-9
25. Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet's membrane. I. Changes with age in normal corneas. Arch Ophthalmol. 1982;(12)100:1942- 1947. https://doi.org/10.1001/archopht.1982.01030040922011
26. Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet's membrane. II. Aphakic bullous keratopathy. Arch Ophthalmol 1982;100(12):1948- 1951. https://doi.org/10.1001/archopht.1982.01030040928012
27. Warin III GO. Posterior collagenous layer of the cornea. Ultrastructural classification of abnormal collagenous tissue posterior to Descemet's membrane in 30 cases. Arch Ophthalmol. 1982;100(1):122-134. https://doi.org/10.1001/archopht.1982.01030030124015
28. Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Gent. 2001;10(21):2415-2423. https://doi.org/10.1093/hmg/10.21.2415
29. KrachmerJH, Purcell JJ Jr.,Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol. 1978;96(11):2036-2039. https://doi.org/10.1001/archopht.1978.03910060424004
30. Holden BA, Williams L, Zantos SG. The etiology of transient endothelial changes in the human cornea. Invest Ophthalmol Vis Sci 1985;26:1354–1359.
31. Bonanno JA. Molecular mechanism underlying the corneal endothelial pump. Exp Eye Res. 2012;95(1):2-7. https://doi.org/10.1016/j.exer.2011.06.004
32. Lindstrom RL. Advances in corneal preservation. Trans Am. OphthalmolSoc1990;88:555-648.
33. Srinivas SP. Dynamic regulation of barrier integrity of the corneal endothelium. Optom Vis Sci. 2010;87(4): E239-E254. https://doi.org/10.1097/ OPX.0b013e3181d39464.
34. Bourne WM, Brubaker RF. Decreased endothelial permeability in transplanted corneas. Am J Ophthalmol. 1983; 96(3):362-367. https://doi.org/10.1016/S0002-9394(14)77828-6
35. Bourne WM, Nelson L R, Buller CR, Huang PT, Geroski DH, Edelhauser HF. Long-term observation of morphologic and functional features of cat corneal endothelium after wounding. Invest Ophthalmol Vis Sci. 1994; 35(3):891-899.
36. Bourne WM. Functional measurements on the enlarged endothelial cells of corneal transplants. Trans Am Ophthalmol Soc. 1995;93:65-79.
37. Rao GN, Aquavella JV, Goldberg SH et al. Pseudophakic bullous keratopathy: Relationship to preoperative corneal endothelial status. Ophthalmology 1984;91(10):1135–1140. https://doi.org/10.1016/S0161-6420(84)34168-9
38. Bourne WM. Clinical estimation of corneal endothelial pump function. Trans Am Ophthalmol Soc. 1998; 96: 229–242.
39. Kaye GI, Tice LW: Studies on the cornea. V. Electron microscopic localization of adenosine triphosphatase activity in the rabbit cornea in relation to transport. Invest Ophthalmol Vis Sci 1966;5(1): 22-32.
40. Geroski DH, Edelhauser HF. Quantitation of Na/k ATPase pump sites in the rabbit corneal endothelium. Invest Ophthalmol Vis Sci 1985; 25(9):1056-1060, 1984
41. O'Neal MR, Polse KA. Decreased endothelial pump function with aging. Invest Ophthalmol Vis Sci 1986;27(4): 457-463.
42. Polse KA, Brand R, Mandell R, et al: Age differences in corneal hydration control. Invest Ophthalmol Vis Sci 1989; 30 (3):392-399.
43. Rao GN, Shaw EL, Arthur EJ, and Aquavella JV: Endothelial cell morphology and corneal deturgescence. Ann Ophthalmol 11:885, 1979.
44. McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices and new intraocular drugs and solutions. Cornea 2008;27(1):1-16. https://doi.org/10.1097/ICO.0b013e31815892da
45. Wörner CH, Olguín A, Ruíz-García JL, Garzón-Jiménez N. Cell Pattern in Adult Human Corneal Endothelium CH.PLoS One, 2011: 6 (5):1-5( e19483).
46. Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N.Effects of long-term extended contact lens wear on the human cornea. Invest. Ophthalmol. Vis Sci.1985; 26(11):1489-1501.
47. Carlson KH, Bourne WM, Brubaker RF. Effect of long-term contactlens wear on corneal endothelial cell morphology and function. InvestOphthalmol Vis Sci 1988;29 (2):185–193.
48. MacRaeSM, Matsuda M, Shellans S. Corneal endothelial changesassociated with contact lens wear. CLAO J 1989;15:82–87.
49. MacRae SM, Matsuda M, Phillips DS. The long-term effects of polymethylmethacrylate contact lens wear on the corneal endothelium. Ophthalmology 1994;101(2):365–370. https://doi.org/10.1016/S0161-6420(94)31327-3
50. Schoessler JP, Orsborn GN. A theory of corneal endothelial polymegethism and aging. Cur Eye Res.1987; 6 (2):301-306. http://dx.doi.org/10.3109/02713688709025182
51. Bergmanson JP. Histopathological analysis of corneal endothelial polymegethism. Cornea. 1992;11(2):133-142. https://doi.org/10.1097/00003226-199203000-00007
52. Shaw EL, Rao GN, Arthur EJ, Aquavella JV. The functional reserve of corneal endothelium. Ophthalmology. 1978;85(6):640-649. https://doi.org/10.1016/S0161-6420(78)35634-7
53. Laing RA, Sanstrom MM, Berropsi AR, Leibowitz HM. Changes in the corneal endothelium as a function of age. Exp Eye Res 1976;22(6):587-594. https://doi.org/10.1016/0014-4835(76)90003-8
54. Bourne WM, Richard F, Brubaker RF, O'Fallon M. Use of air to decrease endothelial cell loss during intraocular lens implantation. Arch Ophthalmol. 1979; 97(8):1473-1475.https://doi.org/10.1001/archopht.1979.01020020135009
55. PoIse KA, Brand RJ, Cohen SR, Guillon M. Hypoxic effects on corneal morphology and function. Invest Ophthalmol Vis Sci. 1990;31(8): 1542-1554.
56. Nieuwendaal CP, Odenthal MT, Kok JH, Venema HW, Oosting J, Riemslag FC, Kijlstra A. Morphology and function of the corneal endothelium after long-term contact lens wear. Invest Ophthalmol Vis Sci. 1994;35 (7):3071–3077.
57. Bruinsma M, Lie JT, Groeneveld-van Beek EA, Liarakos VS, van der Wees J, Melles GR. Are polymegethism, pleomorphism, and "poor swelling" valid discard parameters in immediate postmortem evaluation of human donor corneal endothelium? Cornea. 2013;32(3):285-9. https://doi.org/10.1097/ICO.0b013e318253b1a6.
58. Doughty MJ. The ambiguous coefficient of variation: Polymegethism of the corneal endothelium and central corneal thickness. International Contact Lens Clinic. 1990:17(9)240-248. https://doi.org/10.1016/0892-8967(90)90064-M
59. Bourne WM. Cellular changes in transplanted human corneas. Cornea 2001;20(6):560-569. https://doi.org/10.1097/00003226-200108000-00002
60. Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci 2003;44:3326–3331.
61. Nishimura JK, Hodge DO, Bourne WM. Initial endothelial cell density and chronic endothelial loss rate in corneal transplants with late endothelial failure. Ophthalmology1999;106(10):1962-1965. https://doi.org/10.1016/S0161-6420(99)90409-8
62. Bourne WM, Nelson LR, Maguire LJ et al. Comparison of Chen medium and Optisol-GS for human corneal preservation at 4ºC. Results of transplantation. Cornea 2001;20(7):683-686. https://doi.org/10.1097/00003226-200110000-00003
63. Pels E, Schuchard Y. Organ-culture preservation of human corneas. Doc Ophthalmol. 1983;56(1-2):147–153. https://doi.org/10.1007/BF00154722
64. Bourne WM. Endothelial cell survival on transplanted human corneas preserved at 4 C in 2.5% Chondroitin Sulfate for one to 13 Days. Am J Ophthalmol. 1983;103(3):382-386
65. Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology 1998;105(10):1855-1865. https://doi.org/10.1016/S0161-6420(98)91030-2
66. Borderie VM, Scheer S, Touzeau O et al. Donor organ cultured corneal tissue selection before penetrating keratoplasty Br J Ophthalmol. 1998;82(2):382-388. https://doi.org/10.1136/bjo.82.4.382
67. Lass JH, Sugar A, Bentez BA, et al.; Cornea Donor Study Investigator Group. Edothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128(1):63-69. https://doi.org/10.1001/archophthalmol.2010.128.63

Funding: No specific financial support was available for this study.
CEP Approval: Not applicable.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
January 8, 2018.
Accepted on:
March 15, 2018.