Carlos Augusto Moreira Neto
DOI: 10.17545/e-oftalmo.cbo/2015.35
The American Joint Committee on Cancer (AJCC) classification is in its seventh edition. In addition to universal tumor staging (TNM), this classification is very relevant in the field of ocular oncology, which involves iris, ciliary body, and choroidal melanoma. It aimed to separate uveal melanoma into anatomical stages to provide information regardingthe prognosis for metastasis and death.
The AJCC's classification of posterior uveal melanoma involves melanoma categorization and staging. Categorization includes measurements of the tumor thickness andtumor base. It defines which of the four previously designated size groups the tumor fits into: T1, T2, T3, or T4 (TABLE 1). The staging process involves the categorization results and information regardinglymph nodes (N) and metastasis (M) to define which of the four anatomical stages the tumor fits into: I, II, III, or IV (TABLE 2).
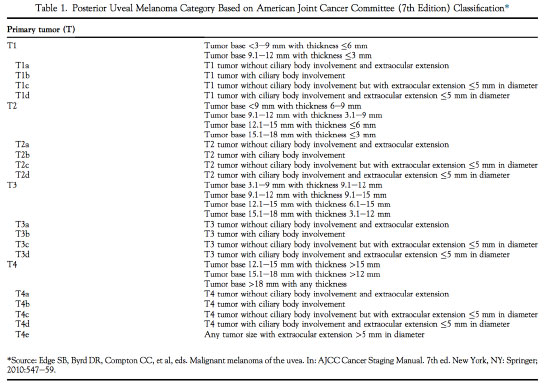
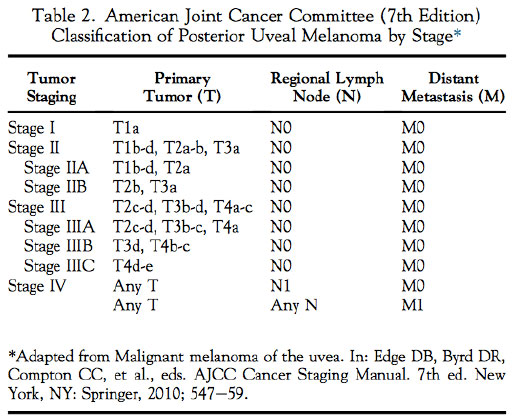
The present article1 sought to explore the predictive value of the AJCC's anatomical stage classification system for metastasis and death by posterior uveal melanoma.
Based on data from the Ocular Oncology Service at the Wills Eye Hospital, 7731 patients with a diagnosis of ciliary body or choroidal melanoma between 1970and 2008 were considered. There wasadequate tumor information regardingall patients, which enabled retrospective classification of uveal melanoma using AJCC categorization (7th edition). Basal diameter was estimated using binocular indirect ophthalmoscopy and ultrasonography, and tumor thickness was measured using ultrasonography. Varioustreatment options were used with these patients, including laser photocoagulation, transpupillary thermotherapy, plaque radiotherapy, local resection, enucleation, and exenteration. Furthermore, systemic monitoring and screening for metastasis were performed.
Out of the 7731 patients, 2767 (36%), 3735 (48%), 1220 (16%), and 9 (<1%) were classified as having stage I, II, III, and IV tumors, respectively.
Following data analysis, it was estimated that, of the patients with 10 and 20 years of follow-up, the respective probability of metastasis was 12% and 20% for stage I patients, 29% and 44% for stage II patients, 61% and 73% for stage III patients (p<0.001), and 100% among stage IV patients after 1 year of follow-up.
Meanwhile, the respective estimates of melanoma-related death at 10 and 20 years after diagnosis were 6% and 8% for stage I patients, 15% and 24% for stage II patients, 39% and 53% for stage III patients, and 100% among stage IV patients after 1year of follow-up (p<0.001).
The predictive factors for metastatic disease and death in cases of stage I melanoma include a large tumor base and an increase in tumor thickness. Aging, increase in tumor thickness, a large tumor base, tumor pigmentation, a mushroom configuration, and associated subretinal fluid were predictive factors among stage II patients. Stage III factors included not only the abovementioned factors but also an inferior tumor location.
A recent publication by the AJCC Ophthalmic Oncology Task Force mentioned a study using a cohort of 3809 patients from 10 international ocular oncology centers. The estimates ofmetastasis-free survival 10 years after diagnosis were found to be 94% (stage I), 78% (stage II), and 56% (stage III).
Thus, the analysis of 7731 patients with posterior uveal melanoma revealed the AJCC's clinical classification to be predictive of metastatic disease. Briefly, this classification may be used to select patients at high risk for metastasis for treatment protocols that focus on the prevention of metastatic events. Furthermore, itshows the importance of early detection and disease management.
REFERENCES
1. Shields CL, Kaliki S, Furuta M, Fulco E, Alarcon C, Shields JA. American Joint Committee on Cancer Classification of Uveal Melanoma (Anatomic Stage) Predicts Prognosis in 7,731 Patients: The 2013 Zimmerman Lecture. Ophthalmology. 2015 Jun;122(6):1180-6. http://dx.doi.org/10.1016/j.ophtha.2015.01.026

Funding source: None declared.
Conflict of interest: The author declares to have no conflicts of interest.
Research Ethincs Committee Opinion: N/A.
Received on:
September 15, 2015.
Accepted on:
November 12, 2015.