Carlos Augusto Moreira Júnior1; Carlos Augusto Moreira Neto2
DOI: 10.17545/e-oftalmo.cbo/2015.50
ABSTRACT
The authors provide a broad revision of macular holes, which have a prevalence of 7.8 cases per 100 thousand people. They discuss the vitreoretinal interface and the tractional forces that occur in this region, all of which cause macular holes. They present the main data for clinical diagnosis. This data includes metamorphopsia and central scotoma, as well as the biomicroscopic aspect of the pathology such as the Watzke-Allen sign, which differentiates pseudoholes from true macular holes. When analyzing the diagnosis via image, they show signs of hyperautofluorescence and hyperfluorescence in angiography, and they place special importance on the optical coherence tomography (OCT) exam, which is the standard exam for the diagnosis of the disease. During the classification of macular holes, they recall the clinical classification described by Gass and correlate it with the current classification of macular holes via OCT. They present the treatment via pars plana vitrectomy, showing that, with an appropriate technique the holes are closed in approximately 100% of the cases and at least 65% of patients have their vision improved to better than 20/40. In the end, they discuss the surgical complications and the differential diagnosis of pathology.
Keywords: Retinal Perforations. Tomography, Optical Coherence.
RESUMO
Os autores fazem uma ampla revisão sobre os buracos maculares, mostrando que a prevalência é de 7,8 casos por 100 mil pessoas. Discorrem sobre a interface vítreorretiniana e sobre as forças tracionais que ocorrem nessa região, dando origem ao buraco macular. Apresentam os principais dados para o diagnóstico clínico que são a metamorfopsia e escotomas centrais, bem como o aspecto biomicroscópico da patologia como o sinal de Watzke-Allen, que diferencia o pseudoburaco do buraco macular verdadeiro. Quando analisam o diagnóstico por imagem mostram os sinais de hiperautofluorescência e hiperfluorescência na angiografia, dando importância especial para o exame de tomografia de coerência óptica (OCT), que é o exame padrão para o diagnóstico da doença. Quando classificam os buracos maculares, lembram a classificação clínica descrita por Gass e fazem uma correlação com a atual classificação dos buracos maculares pelo OCT. Apresentam o tratamento com a cirurgia de vitrectomia via pars plana, mostrando que com a técnica adequada, o fechamento desses buracos ocorre em quase 100% dos casos, sendo que em pelo menos 65% deles a visão fica melhor que 20/40. Ao final tratam das complicações cirúrgicas e do diagnóstico diferencial da patologia.
Palavras-chave: Perfurações Retinianas. Tomografia de Coerência Óptica.
INTRODUCTION
The posterior vitreous cortex and the internal limiting membrane (ILM) of the retina are adhered to its interface through a complex of macromolecules composed of fibronectin, laminin, and other extracellular components (1,2). An inappropriate or incomplete separation of the vitreous can result in abnormal detachment, causing pathological changes to the vitreomacular interface (3). The presence of tractional forces on the macula, which are generated by this inappropriate separation, cause the formation of a macular hole (4,5).
The full thickness macular hole (FTMH) is an anatomic defect that shows solution of continuity of all retinal layers in the foveolar area (figura 1A). High resolution OCT revealed that idiopathic macular holes (those associated with age) are a result of tractional forces existing during the posterior vitreous detachment (PVD) process of the perifoveal area. Such physiopathological phenomena had already been described by Gass (6), even before the existence of optical coherence tomography (OCT).
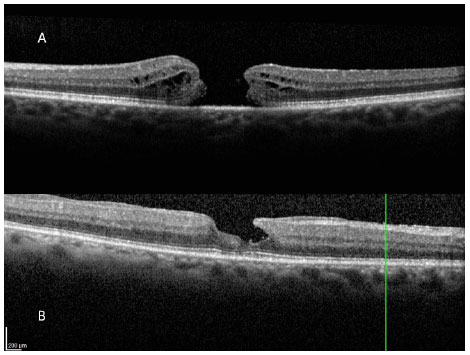
In addition to full thickness macular holes, there are pseudoholes that, in general, occur due to the appearance of an epimacular membrane, which is similar to a full thickness macular hole or a true macular hole, but which does not exhibit interruption of the retinal layers. Lamellar macula holes (figura 1B) exhibit interruption in only one portion of the retinal layers in the foveolar area, unlike cases of full thickness macular holes, in which all retinal layers are interrupted.
The prevalence of full thickness macular holes in the general population is 7.8 cases per 100.000, per year (7). In general, this problem occurs after 60 years of age. The risk of the development of the macular hole in the other eye without manifestation of PVD has been estimated to be between 7% and 12% after 5 years and 17% after 20 years (8). Approximately two thirds of patients are women, and the disease is unilateral in 80% of cases. An increase in the blood level of fibrinogen has been reported as a risk factor for the appearance of the macular hole (9), while the replenishment of estrogen in women reduces the risk of this problem. In myopic patients, macular hole prevalence can reach 6% (10).
CLINICAL DIAGNOSIS
Patients with full thickness macular holes have complaints of loss of central vision, which begins as mild to moderate (20/25 to 20/80), and which can progress to central scotoma with further disease progression (20/200). Depending on the tractional forces on the fovea, various degrees of metamorphopsia can be observed.
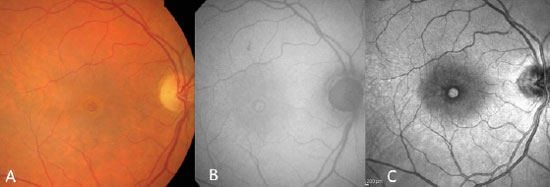
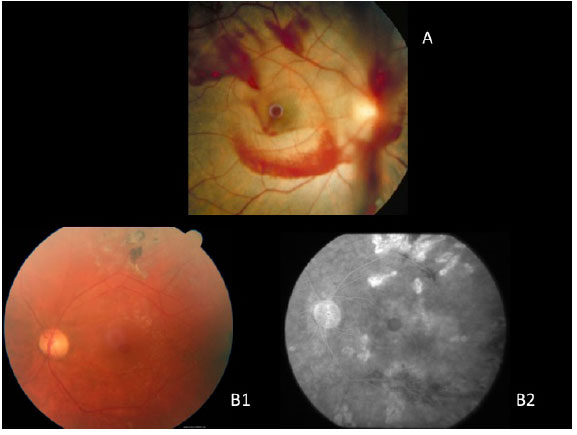
In general, a macular biomicroscopy is sufficient for diagnosis (figura 2A). This exam is able to reveal a well-defined circular lesion in the center of the macula, with yellow deposits at the base, and it frequently shows a sub-retinal liquid halo around the lesion. The Watzke-Allen sign can be used by projecting a narrow beam of light over the center of the macular hole using a Goldmann lens. A patient with a true hole will observe a broken or a thinner beam of light at the location of the hole. On the other hand, patients with a pseudohole or a lamellar hole will see a beam of light with a uniform thickness, though it may be distorted at the location of the pseudohole.
DIAGNOSIS VIA IMAGING
Macular holes were long diagnosed with the aid of fluorescein angiography, which shows the area of hyperfluorescence by default in a window in the foveolar area, due to atrophy of the retinal pigment epithelium (RPE).
Nowadays, autofluorescence and near infra-red images are equally effective at diagnoses, because they do not require contrast injection. True macular holes cause a field of hyperautofluorescence and a hyper near infra-red (figura 2B e C).
Lamellar macula holes appear in autofluorescence exams as a hyperautofluorescent area due to loss of lutein pigment, which appears in higher concentrations in the external plexiform layer of the retina. Because the pseudohole does not cause a loss of retinal tissue, autofluorescence is normal.
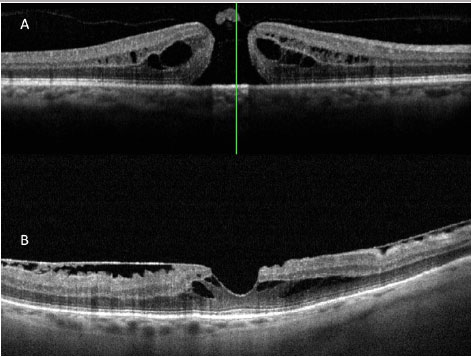
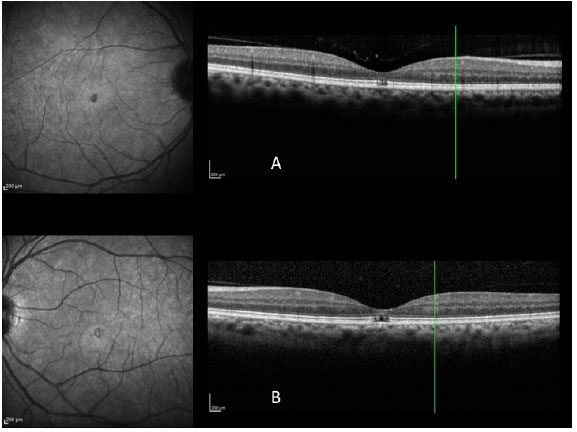
Since the description by Puliafito et al. (11) of the first optical coherence tomography (OCT) images of macular diseases in 1995, OCT has evolved to become a more important exam for the diagnosis and treatment of several macular diseases, especially those that affect the vitreoretinal interface, which is the case of macular holes.
The images obtained with the new high0-resolution spectral domain OCT devices (SD-OCT) are ideal for diagnosis, indication of treatment, and patient follow up (Figura 3 A e B). In the light of these advantages, a new classification system was developed for vitreoretinal interface diseases (3), one which is entirely based on OCT images, in order to classify subtypes of macular holes and to complement Gass's clinical classification (6), which is still widely used.
CLASSIFICATION OF MACULAR HOLES
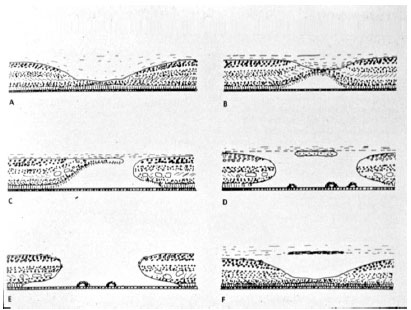
Long before OCT was available, and through biomicroscopical and histological studies of the macula, Gass (6) came to understand the sequence of events that lead to the appearance of idiopathic macular holes. He then created a classification system that is still used today (12), but which was recently replaced by an international system based on OCT findings, which will be described later.
The Gass classification for idiopathic macular holes will be summarized in figura 4. Stage 1, described as an imminent macular hole, is characterized by the tangential contraction of the external pre-foveolar vitreous cortex, causing anterior detachment and serous detachment of the foveola. Stage 2 shows rupture of the vitreous cortex with centrifugal retraction of photoreceptors and a full thickness macular hole less than 400 micron in diameter, which may be central or eccentric. Stage 3 shows a full thickness macular hole with a diameter of more than 400 micron and with an overarching operculum. Finally, stage 4 has the same characteristics of stage 3, but there is separation of the vitreous cortex.
Duker et al. (3) devised a new classification of vitreomacular tractions and macular holes based on the findings of high-resolution optical coherence tomography. According to the researchers, a full thickness macular hole is defined as a foveolar lesion in which all retina layers are interrupted in the internal limiting membrane of the pigment epithelium. The macular hole is primary if caused by vitreous traction and secondary if it is a direct result of pathologic alterations other than th vitreomacular traction.
A high-resolution tomography exam provides a more detailed inference of the margin of the hole, which is generally circular and which may be thicker than the adjacent retinal tissue due to accumulation of intraretinal fluid and its distance from the RPE as a result of tangential vitreous traction.
This tomography classification mainly considers the size of the macular hole, which is directly correlated with the visual result of the surgical treatment of these cases. To define the size of the macular hole, a measurement is taken from the narrowest point between two retinal margins using the measurement function of the OCT with a parallel line to RPE. The macular holes are then classified as small, average, or large.
The small macular holes are the ones with a diameter of less than 250 micron. These holes may be associated with a low rate of spontaneous closure and a high rate of closure via vitrectomy. An average hole has a diameter between 250 micron and 400 micron and a closure rate of more than 90% via vitrectomy. Almost half of the macular holes are large and with a diameter over 400 micron and an equal closure rate through vitrectomy. The state of the vitreous cortex (whether it is adhered to the retina) can also be determined through the use of OCT, Table 1 shows the correlation between the Gass classification and the international classification based on OCT findings.
Secondary macular holes are caused by other diseases and do not have pre-existing or concurrent vitreomacular traction. Causes of secondary macular holes include trauma contusions, electrical discharge, high myopia, macular schisis, macular telangiectasia type 2, and a variety of retinopathies that cause macular edema such as diabetic retinopathy, age-related macular degeneration, vascular occlusions, and uveitis.
TREATMENT:
Approximately 50% of stage 1 holes have a spontaneous solution with the development of vitreous cortex detachment of the macular area. A few full thickness macular holes (approximately 10%) may close spontaneously.
Treatment for full thickness macular holes is typically indicated, and may be enzymatic vitreolysis induction or a vitrectomy surgery.
Ocriplasmin is only used for macular holes than 400 micron in diameter. For smaller holes (less than 250 micron), the rate of hole closure after a single injection of ocriplasmin is high. As for average holes (between 250 and 400 micron), the hole closure rate may be as high as 40% (13). However, experience with this type of treatment is still limited in Brazil.
Since the introduction of the pars plana vitrectomy (PPV) for the treatment of macular holes, introduced by Kelly and Wendel (14) in 1991, the technical improvement of this surgery has been constant and is the procedure of choice for the treatment of macular holes. The anatomical and visual outcomes are very good, especially for recent holes (those with less than 6 months of duration) (Figura 5).
SURGERY TECHNIQUE:
The pars plana vitrectomy (PPV) is typically performed with the use of 25-gauge vitreotomes in order to avoid the need to suture sclerotomies, since the low caliber allows for a suture-free surgery with fast functional recovery. Once the center of the vitreous body is removed, we then proceed with the removal of the hyaloid membrane. For this purpose, the device's suction force must be increased and detachment of the posterior vitreous must be induced. Many surgeons believe that this maneuver is easier to perform with the use of triamcinolone to dye the hyaloid membrane.
After the removal of the hyaloid membrane, it is necessary to remove the internal limiting membrane (ILM) from the retina. This procedure increases the success of surgery to more than 90%. The ILM can be removed through injection of dyes such as indocyanine green or brilliant blue. The use of indocyanine green has decreased in recent years due to reports of toxicity for the retina and optical nerve. Currently, most surgeons use brilliant blue dye for ILM (figure 6). in addition to its ability to provide good coloring of the structure, it is less toxic.
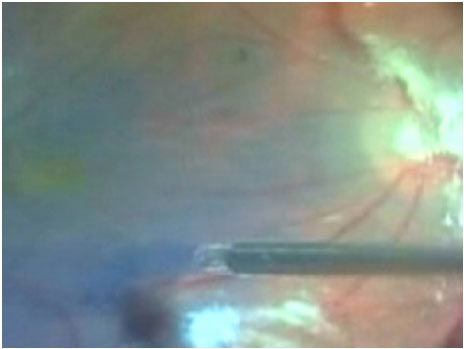
After dying the membrane, a small tear is made in the ILM with the aid of a Tano scraper or a small needle in order to remove the entire ILM from the macular area. The entire posterior pole can then be removed. This procedure avoids the appearance of postoperative epiretinal membranes and allows the Muller cells to close the macular hole. For larger holes (those that are more than 400 micron in diameter), a larger amount of the ILM must be removed.
After removing the ILM, the next step is a complete inspection of the retina to avoid missing retinal tears or other alterations that may compromise the final visual result. This is followed by a full fluid-gas exchange. The procedure is completed with the placement of intraocular gas, which can be SF6 or C3F8, depending on desired buffering time. For very large holes, C3F8 is more frequently indicated, since it remains in the vitreous cavity for a longer period of time and therefore acts for a longer period of time with more buffering force until Muller cells develop at the site.
Since the appearance of cataracts is common during vitrectomies, many surgeons opt to perform phaco-vitrectomy; they remove the crystalline lens and place an intraocular lens, thus achieving full functional recovery of the affected eye.
After surgery, rest with the head facing downward is fundamental. In general, the patient is asked to remain in this position for at least 5 days. This rest period may be longer in cases of larger holes or shorter in cases of small macular holes.
Ocular pressure control and the inflammatory processes are equally important during this period.
RESULTS
The anatomical closure of the hole occurs in approximately 100% of cases, while the functional improvement of vision occurs during the subsequent months. During the sixth month, improvement has been found to manifest as vision superior to 20/40 in 65% of the eyes that undergo surgery.
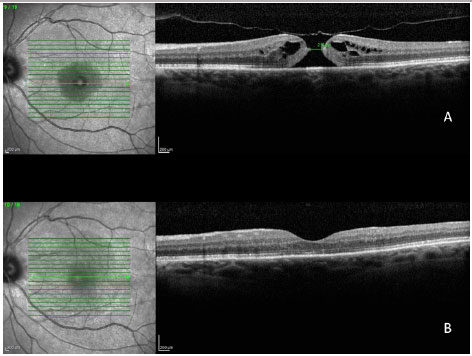
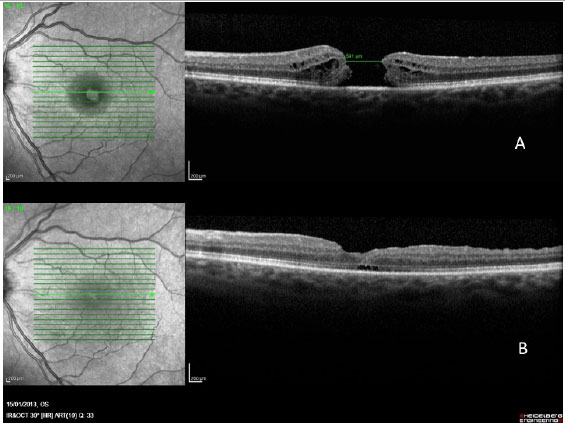
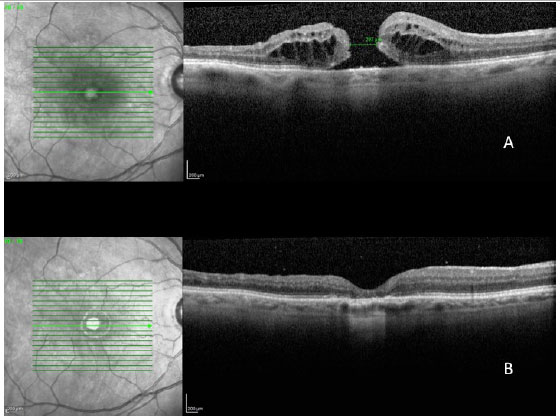
In a 2014 study by Williamson and Lee (15) in which the authors assessed the anatomical and functional result of 351 eyes that underwent surgery for a macular hole, the hole closure rate was found to vary according to it's clinical stage. Holes in stage 2 had a closure rate of 95.8% after surgery (Figura 7); holes in stage 3 closed in 73% of cases (Fiauia 8) and holes in stage 4 closed in 56.3% of cases (Figura 9). The final average visual outcome was of 20/50 in cases of stage 2 holes, 20/110 in cases of stage 3 holes, and 20/145 in cases of stage 4 holes. The authors also found that the hole closure rate was different when the ILM was removed with indocyanine green (73.2%) or with brilliant blue (89.9%). Final average visual acuity for eyes that underwent surgery was 20/100 when indocyanine green was used and 20/70 when brilliant blue was used.
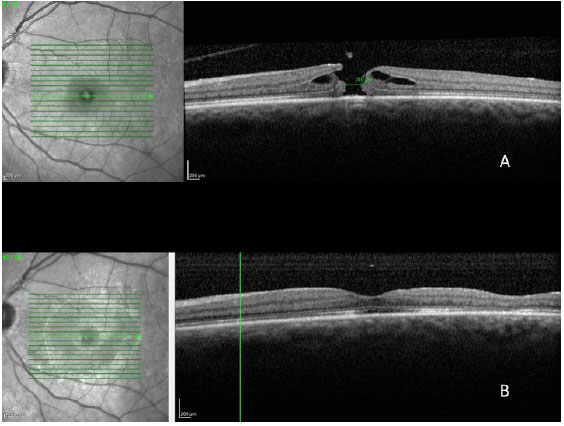
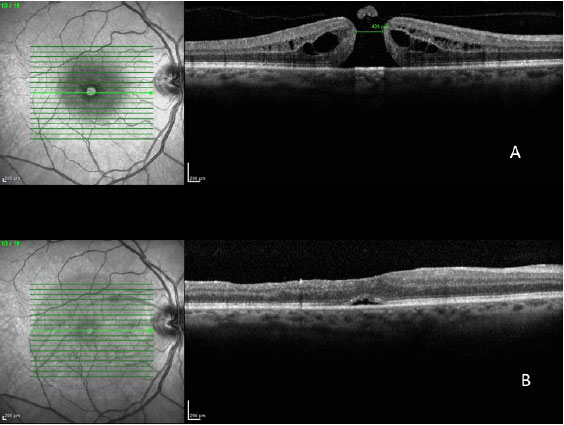
There are several factors that can help to predict the functional outcomes of macular hole cases. Meta-analysis work shows that the macular holes in a more advanced stage and of a larger size have a worse visual result. The appearance time of the symptoms is equally important. Eyes with symptoms during more than one year have significantly worse visual outcomes. Preoperative visual acuity (VA) is also an important factor for the final visual outcome. Eyes with preoperative VA better than 20/200 usually have a final VA of 20/50 or better (16).
COMPLICATIONS
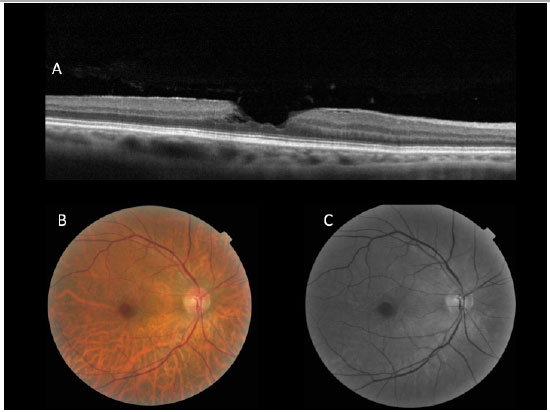
Macular hole non-closure is the most frequent complication, especially in cases of very large holes. Some techniques have been used to treat this problem, such as suspension of the hole margin with pics or placing a drop of blood on the macular hole. In any cases, rest with head facing downward for at least 7 is likely the most important factor in the recovery of this type of complication (Figura 10).
Other complications, such as intraocular infection (endophthalmitis), bleeding, retinal detachment, loss of visual field due to a long period of gas exchange, and intense light over the posterior pole are possible complications of all vitrectomies.
DIFFERENTIAL DIAGNOSIS OF PRIMARY MACULAR HOLES
Secondary macular holes:
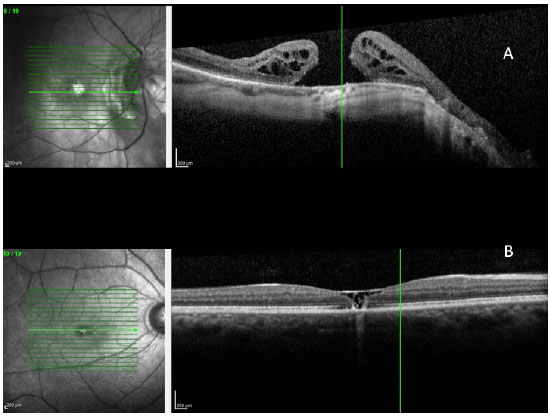
- Pseudohole due to the appearance of the epimacular membrane (Figura 11)
- High myopia - the presence of the posterior staphyloma in high myopia can cause a macular hole with the development of retinal detachment (Figura 12 A)
- Solar retinopathy or electrical discharge (Figura 12 B)
- Trauma contusions can cause the occurrence of a macular hole due to sudden vitreous traction on the macula (Figura 13 A)
- Chronic macular edema can cause a macular hole with significant visual loss; causes include vascular diseases (Figura 13 B C)
- Macular microhole that exhibits interruption in the external layers of the retina and which tends to progress very slowly (Figura 14)
REFERENCES
1 A Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225:89-93. http://dx.doi.ora/10.1007/BF02160337
2. A Russell SR, Shepherd JD, Hageman GS. Distribution of glycoconjugates in the human retinal internal limiting membrane. Invest Ophthalmol Vis Sci. 1991;132:1986-95.
3. A A A Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vítreomacular Traction Study Group classification of vítreomacular adhesion, traction, and macular hole. Ophthalmol. 2013;120:2611-9. http://dx.doi.org/10.1016/j-ophtha.2013.07.042
4. A Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371-82. http://dx.doi.ora/10.1016/i.aio.2009.11.022
5. A Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vítreomacular traction syndrome. Br J Ophthalmol. 2002;86:902-9. http://dx.doi.ora/10.1136/bio.86.8.902
6. A A A Gass JDM. Idiopathic senile macular hole: its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629-39. http://dx.doi.ora/10.1001/archopht.1988.01060130683026
7. A Thapa SS, Thapa R, Paudyal I, et al. Prevalence and pattern of vitreo-retinal diseases in Nepal: the Bhaktapur glaucoma study. BMC Ophthalmol. 2013;13:9. http://dx.doi.ora/10.1186/1471-2415-13-9
8. A Steel DHW and Lotery AJ. Idiopathic vítreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye. 2013;27:S1-S21. http://dx.doi.ora/10.1038/eve.2013.212
9. A Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1994;118:754-61. http://dx.doi.ora/10.1016/S0002-9394(14)72555-3
10. A Coppe AM, Ripandelli G, Parisi V, Varano M, and Stirpe M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmol. 2005;112:2103-9. http://dx.doi.ora/10.1016/j.ophtha.2005.06.028
11. A Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmol. 1995;102:217-29. httP://dx.doi.ora/10.1016/S0161-6420(95)31032-9
12. A Gass JDM. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752-9. httP://dx.doi.ora/10.1016/S0002-9394(14)72781-3
13. A Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, Haller JA; MIVI-TRUST Study Group. Enzymatic vitreolysis with ocriplasmin for vítreomacular traction and macular holes. N Engl J Med. 2012;16;367(7):606-15. http://dx.doi.ora.br/10.1056/NEJMoa1110823
14. A Kelly NE, Wendel RT: Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991 ;109:654-9. http://dx.doi.org/10.1001/archopht.1991.01080050068031
15. A Williamson TH, Lee E. Idiopathic macular hole: analysis of visual outcomes and the use of indocyanine green or brilliant blue for internal limiting membrane peel. Graefes Arch Clin Exp Ophthalmol. 2014 Mar;252(3):395-400. http://dx.doi.org/10.1007/s00417-013-2477-2
16. A Kusuhara S & Negi A. Predicting Visual Outcome following Surgery for Idiopathic Macular Holes. Ophthalmologica. 2014;231:125-132. http://dx.doi.org/10.1159/000355492


Source of funding: Nothing to declare.
Opinion of the Research Ethics Committee (EC): Not applicable.
Conflict of interests: None do declare.
Received on:
April 25, 2016.
Accepted on:
May 16, 2016.