Vitor Souza Magalhães; Milena Souza Ribeiro Santos; Marina Viegas Moura Rezende Ribeiro
DOI: 10.17545/eOftalmo/2024.0013
ABSTRACT
PURPOSES: Dry eye syndrome is defined as a multifactorial disease of the tears and the ocular surface, which triggers symptoms such as visual discomfort, visual instability, and lacrimal defects. Despite the optimization of conventional therapies, some patients are refractory, persisting with signs and symptoms. However, such patients can benefit from additional therapies that use blood components and blood derivatives. This study aimed to conduct an integrative review of the indexed literature over the last decade to verify and examine the knowledge available regarding the treatment of dry eye disease using blood components and blood products.
METHODS: The study was an integrative review, and searches were performed on MEDLINE, LILACS, SciELO, and EMBASE databases using the search terms: "dry eye syndrome," "dry eye disease," "dry eye treatment," "blood," "serum," and "platelet-rich plasma" as well as their equivalent terms in Portuguese. Overall, 597 potentially eligible publications were identified, and 16 randomized and non-randomized clinical trials that met the inclusion criteria were selected.
RESULTS: Most of the studies analyzed (9) included treatment with autologous serum, and seven studies evaluated platelet concentrates.
CONCLUSION: Therapy with blood components and blood derivatives has been proven to be safe and effective; moreover, it is a valid option for patients who are refractory to traditional therapies. It is crucial to perform further clinical studies with a larger sample size to better establish and standardize the processes and formulations that can be used in the treatment of dry eye syndrome.
Keywords: Dry eye syndrome; Dry eye disease; Dry eye treatment; Blood; Serum; Platelet-rich plasma.
RESUMO
OBJETIVOS: A doença do olho seco é definida como uma doença multifatorial das lágrimas e da superfície ocular que desencadeia sintomas de desconforto, instabilidade visual e alteração lacrimal. Apesar da maximização das terapêuticas convencionais, alguns pacientes são refratários, persistindo com sinais e sintomas, se beneficiando de terapias adicionais, como os hemocomponentes e hemoderivados. O objetivo foi realizar uma revisão integrativa da literatura indexada nos últimos 10 anos, verificar e examinar o conhecimento descrito sobre o tratamento da doença do olho seco utilizando hemocomponentes e hemoderivados.
MÉTODO: O estudo foi definido como uma revisão integrativa, sendo feito buscas nas fontes de dados MEDLINE, LILACS, SciELO e EMBASE, utilizando os descritores: "síndrome do olho seco", "doença do olho seco", "tratamento do olho seco", "sangue", "soro" e "plasma rico em plaquetas". Foram identificados 597 publicações potencialmente elegíveis, selecionando-se, ao final, 16 ensaios clínicos randomizados e não randomizados que atendiam aos critérios de inclusão.
RESULTADOS: A maioria dos estudos analisados (9) abordou o tratamento com soro autólogo, sete estudos avaliaram com concentrado de plaquetas.
CONCLUSÃO: A terapêutica com hemocomponentes e hemoderivados mostrou ser segura e efetiva, sendo uma opção válida para os pacientes refratários às terapias tradicionais. Ressalta-se a importância da realização de novos estudos clínicos, com maior amostra, para melhor estabelecimento e padronização dos processos e formulações a serem utilizadas no tratamento da doença do olho seco.
Palavras-chave: Síndrome do olho seco; Doença do olho seco; Tratamento do olho seco; Sangue; Soro; Plasma rico em plaquetas.
INTRODUCTION
Dry eye syndrome (DES) affects 5%-34% of the global population1, and it is defined by the Dry Eye Workshop as a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. Furthermore, it is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface2. Although substantial progress has been made in recent years in the field of its pathophysiology and treatment, ongoing therapeutic study of the disease is necessary owing to its chronic nature as well as for establishing specific etiological treatment3.
Tears play an essential role in maintaining the health of the ocular surface as their components play an active role in the regulation, proliferation, differentiation, and maturation of the epithelium4. Conventional treatment for DES includes artificial tears (ATs), topical corticosteroids, ciclosporin-A, therapeutic contact lenses, and punctal occlusion5. ATs, although widely available, lack the biological properties that promote ocular surface renewal and immune defense6. Despite the optimized use of these therapies, some patients are refractory, with persistent signs and symptoms of severe DES. These patients benefit from additional therapies such as the use of blood components and blood derivatives from the processing of donor blood7.
Autologous serum eye drops (ASEs) were first used in 1984 by Fox et al. to treat DES in patients who were refractory to the use of ATs8. ASEs have osmolarity and biomechanical properties similar to natural tears and are preservative-free, which reduces the incidence of allergy and toxicity9. Ever since, ASEs have been successfully used to treat severe DES related to various conditions, such as Sjögren's syndrome (SS), superior limbic keratoconjunctivitis, Stevens-Johnson syndrome, and others10. SS is a systemic autoimmune disease characterized by chronic inflammation resulting in xerostomia and DES. It is classified as primary (absence of another connective tissue disease) and secondary (associated with other systemic diseases such as lupus, rheumatoid arthritis and scleroderma)11. ASEs exhibit therapeutic effects because their pH and osmolarity are similar to those of the tear film. Its mechanism of action is based on lubricating properties and concentration of vitamins A and E, growth factors, fibronectin, and cytokines capable of promoting cell trophism within the epithelium, improving its regeneration12. ASEs also promote epithelial improvement by binding to and neutralizing inflammatory cytokines. ASEs also contain bactericidal components, lysozyme, lactoferrin, and immunoglobulins, thus reducing the risk of contamination and infection in patients with dry eye3.
Use of platelet-rich plasma (PRP) eye drops has an anti-apoptotic effect on corneal stromal cells and has become a valid strategy used in the management of various ocular surface disorders, including corneal ulcers and persistent epithelial defects secondary to moderate and severe DES13. Recently, use of PRP has been highlighted due to its advantages over ASEs, such as the higher amount of epithelial growth factors and vitamin A, and because it is free of inflammatory cytokines14.
Complications with the use of blood components and blood derivatives in the treatment of DES are rare, specifically when safety protocols for preparation and storage are properly followed. If such complications do occur, they are well tolerated, with patients developing ocular discomfort, mild epitheliopathy, bacterial conjunctivitis, or eyelid eczema15. Therapy may be contraindicated for some groups, such as in children, the elderly, patients with severe systemic bacterial infection, acute autoimmune disease, or those with a fear of phlebotomy. It may also be contraindicated for patients with restrictions on autologous blood donation, such as those with congestive heart failure, severe aortic stenosis, heart attack, or stroke in the last 6 months, angina, cyanotic disease, infection, or those using antibiotics16.
METHODS
An integrative literature review was conducted, thoroughly searching and evaluating studies published on the proposed theme. This research method aims to analyze the knowledge already available in existing research on a given topic17.
Data were collected between May and October 2022, and the search strategy was applied in Portuguese and English, with the aim of answering the following question: "What blood component and blood product treatments are available for dry eye disease?". A search was performed for randomized and non-randomized clinical trials published between 2012 and 2022 using MEDLINE, LILACS, SciELO, and EMBASE databases. The search strategy used the descriptors (síndrome do olho seco; doença do olho seco OR tratamento do olho seco) AND (sangue OR soro OR plasma rico em plaquetas) as well as their respective English counterparts (dry eye syndrome OR dry eye disease OR dry eye treatment) AND (blood OR serum OR platelet-rich plasma) constructed from descriptors available in the Health Sciences Descriptors (DECS) terminology, articulated by the Boolean operators "AND" and "OR."
After searching the aforementioned databases, the following inclusion criteria were adopted: randomized and non-randomized clinical trials published in Portuguese and English; related to the treatment of dry eye with blood components or blood derivatives; human trials; published between 2012 and 2022; available in full text in electronic format; and published in national and international journals. Moreover, the exclusion criteria were as follows: treatment with blood components or blood derivatives not related to DES; publications not specifically related to the keywords; and publications in languages other than English or Portuguese.
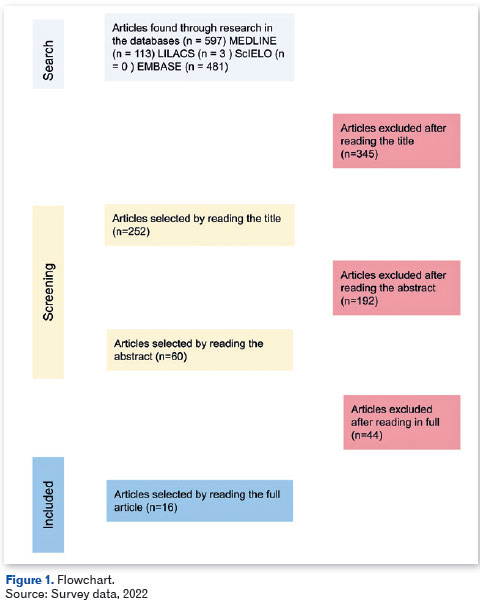
The flowchart (Figure 1) depicts the articles that were chosen, starting with a title-only selection of papers that did not include review articles, case reports, experience reports, and other non-quantitative research that could not be included in an integrative review. After this initial screening, 60 articles that answered the guiding question and fit the description of the research were chosen after thoroughly reading the abstract Of these, 16 were finally chosen after reading the entire article. Lists of citations and bibliographical references pertinent to the objective of the study were also researched.
RESULTS
Data collection took place during the first and second semesters of 2022. The selected articles were read, and the information was extracted and organized using the following variables: author/year, type of study, place of publication, therapy, and results, which is depicted in table 1.
Seventeen adult patients with severe bilateral DES took part in a 3-month clinical trial, using 20% ASEs no more than 12 times a day, 15 min after the application of ATs. There was a significant improvement in the clinical examination using the Tear Breakup Time (BUT) test (1.7 s–2.2 s) and the Schirmer test (ST) (1.1 mm–2.2 mm). In regard to subjective symptoms, there was a gradual, statistically significant improvement in the sensation of dryness, discomfort, sensation of a foreign body in the eye, and photophobia. Clinical evaluation was performed before therapy and at the end of the 3 months, depicting a substantial improvement in 72% of the patients examined who used ASEs, specifically in relation to the sensation of a foreign body in the eye18.
Cho et al. compared the efficacy of 100% ASEs with three different dilutions of ASEs (50% + NS0.9%, 50% + AT, and 50% + ceftazidime) in the treatment of 85 patients divided into three groups: Group I-SS carriers, Group II-Non-SS carriers, and Group III-Persistent epithelial defects. All groups underwent an assessment for the speed of epithelial repair using the measurement of the area (mm2/day) in a slit lamp. In SS carriers, undiluted ASEs had a significantly higher repair speed when compared to the other three dilutions of 50% ASEs12.
A randomized clinical trial conducted by Celebi et al. evaluated the efficacy of 20% ASEs compared to AT without preservatives in patients with severe DES. This study included 20 patients (18 women and 2 men) who were refractory to conventional treatment, and who showed a significant improvement in symptoms, with no adverse effects, after one month of using ASEs, compared to the AT group. This improvement was evidenced by quantitative analysis of the ST, the break-up (TBUT), and the Ocular Surface Disease Index (OSDI) -Allergan questionnaire4.
Hwang et al. evaluated the effects on 20 patients with primary SS and 14 with secondary SS over 4 weeks of therapy with 50% ASEs. There was a significant improvement in symptoms in primary SS, which was not evident in secondary SS. Moreover, analysis of the concentration of the following proinflammatory cytokines TNF-a, IL-1b, IL-6, and IL-8 resulted in higher values in secondary SS. Thus, it has been suggested by the authors that ASEs are not effective in treating DES in patients with secondary SS11.
A study conducted by Yilmaz et al. compared the effectiveness of 40% ASEs with ATs in the treatment of patients with DES secondary to the use of systemic retinoic acid (isotretinoin). Patients with other previous ocular surface pathologies were excluded. Isotretinoin reduces the function of the meibomian gland that produces the lipid component of tears, leading to DES. In this randomized clinical trial, 40% ASEs proved to be an effective alternative compared to ATs without preservatives1.
Some patients have logistical limitations to the use of their own blood to prepare the ASEs (e.g., inability to travel to the collection center or sample processing time), and others may have clinical factors that pose a barrier (e.g., difficulty with venous access, low hemoglobin levels, fear of needles, or the patient's age). Thus, van der Meer et al. conducted a randomized clinical trial with 15 patients with severe DES, comparing the efficacy and tolerability of using eye drops produced from autologous serum and allogeneic serum (from other donors). The authors observed no significant differences, with comparable efficacy and tolerability19.
Ribeiro et al. evaluated the efficacy of autologous PRP eye drops, applied four times a day for 1 month in the symptomatic dry eyes of 12 diabetic patients who were refractory to conventional therapies. All patients showed improvement in symptoms and signs in the tests adopted-ST and TBUT16. Six months after PRP eye drops, Ribeiro et al. performed a novel evaluation of the persistence of the effects of PRP therapy. Patients in that study used the ATs of their choice for 5 months after an initial 1-month therapy with PRP eye drops. The OSDI and TBUT scores showed higher values than the baseline 6 months before therapy, which corroborates the hypothesis of a prolonged effect14.
In the randomized clinical trial conducted by Garcia-Conca et al., 84 patients with hyposecretory dry eye were divided into two groups: 44 treated with PRP and 39 with AT. Changes in ST and TBUT were assessed 30 days after treatment. The group treated with PRP eye drops exhibited superiority in the following aspects when compared to the group using AT: reduction in symptoms, visual improvement, and reduction in hyperemia and improvement in ST20.
The use of 20% PRP compared to 20% ASEs, with 10 drops in each eye for 1 month, in a group of 10 patients in a randomized clinical trial conducted by Metheetrairut et al. revealed a significant improvement in ST and visual acuity test for those that used 20% PRP. No adverse effects were reported by the patients in the study21.
Platelet concentrate injection (PCI) therapy in the lacrimal glands (LG) was shown to be superior to AT in the treatment of glandular dysfunction in a randomized clinical trial with 13 patients diagnosed with SS, in which PCI was applied at regular intervals, on days 0, 30, 60, and 90. Tear volume increased significantly when compared to the AT group13.
A study conducted by Allam randomized 20 patients (40 eyes) into two groups, the first group received PCI in the LG at regular interviews, on days 0, 30, 60, and 90. The second group received 50% ASEs five times a day. At the end of the 12-week study, both therapies were comparable in terms of effectiveness. Although PCI is a painful therapy, this issue was addressed by injecting anesthetic into the LG region 5 minutes before the procedure. Although no complications were observed in either groups, four eyes using ASEs showed mild irritation. In contrast to ASE therapy, an advantage of PCI is that it does not depend on the patient's cooperation for storage, which could potentially lead to contamination if not done according to prior instructions6.
Mohammed et al. performed a study with 28 eyes to compare PCI in LG with AT. This study was similar to the one performed by Avila et al. as both included only patients with severe DES secondary to SS13. The patient's own eye was used as a control group, i.e., in the same patient, one eye received PCI and the contralateral eye received AT. After 3 months, analysis showed significantly greater efficacy in favor of ICP22.
DISCUSSION
Autologous serum eye drops (ASE)
ASEs are produced from the patient's own blood and are similar to natural tears in terms of their biochemical and epitheliotropic aspects. The use of ASEs aims to treat patients who are refractory to conventional treatment for dry eyes or other epitheliopathies12. ASEs are obtained by centrifugation, which separates the blood from the serum. The serum is then diluted and can be stored for up to 6 months at −30°C9. There is much discussion regarding what concentration should be used in the preparation of ASEs, as TGF-ß, a molecule present in serum that has an antiproliferative effect23, has a concentration five times higher than that found in human tears9. The lack of consensus on the dilution of ASEs leads to the use, in treatment studies, of formulations that vary in concentration ranging from 20% to 100%11.
Platelet-rich plasma (PRP) eye drops
PRP have been used successfully to treat pathologies in various fields of medicine, particularly ophthalmology, orthopedic, and maxillofacial and plastic surgery24.
Platelet concentrate injection (PCI)
PCI is a therapy used to regenerate tissues due to its richness in growth factors, which stimulate the proliferation and regeneration of stem cells25. In ophthalmology, it has been successfully used to increase the production of dysfunctional LG secondary to pathologies such as SS22. The LG is crucial for the health of the ocular surface, and therefore, pathologies that affect its functioning can lead to aqueous deficiency and loss of ocular surface homeostasis26. The tear film is made up of three layers: the inner one composed of mucin, the outer one composed of lipid, and the aqueous intermediate layer (produced mainly in the LG), which when insufficient, is one of the main causes of DES27.
The studies mentioned in the results address different therapies for the treatment of DES and its variations, including the use of ASEs and PRP eye drops, and PCI. The comparative analysis suggests that the choice of therapy for the treatment of DES may depend on the underlying cause, the severity of the disease, and patient preferences. Moreover, ASEs, PRP, and PCI seem to be effective options in different clinical scenarios, with each having specific benefits. PRP eye drops work effectively in the treatment of diabetic patients, whereas PCI are effective in the treatment of lacrimal gland in patients with severe DES secondary to SS.
Each therapeutic approach has its advantages and disadvantages, and the choice should be personalized based on the patient's clinical assessment. Research into new treatments for DES has increased substantially in recent years. The most significant change is the search for therapies that can reverse the underlying etiology, such as inflammation and aqueous deficiency, as opposed to just treating the symptoms. Innovative therapies are options available to patients who are refractory to traditional therapies. The future goal is increasingly to refine therapies that bring definitive resolution rather than transient symptomatic relief.
Although DES has a multifactorial etiology, results of the clinical trials analyzed suggest that treatments with blood components and blood derivatives, such as ASEs, PRP, and PCI are promising options for patients with DES, especially those refractory to conventional treatments. The choice between these therapies must be individualized, considering the etiology of DES, the severity of the condition, and the patient's preferences. Additionally, the continuous development of innovative therapies seeks to offer more effective and lasting solutions to DES, with the ultimate goal of resolving the underlying causes rather than just temporarily relieving the symptoms.
REFERENCES
1. Yılmaz U, Küçük E, Koç Ç, Gökler E. Comparison of Autologous Serum Versus Preservative Free Artificial Tear in Patients with Dry Eyes Due to Systemic Isotretinoin Therapy. Current Eye Research. 2017;42(6):827-31.
2. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. The Ocular Surface. 2017;15(3):276-83.
3. Rodríguez Calvo-de-Mora M, Domínguez-Ruiz C, Barrero-Sojo F, Rodríguez-Moreno G, Antúnez Rodríguez C, Ponce Verdugo L, et al. Autologous versus allogeneic versus umbilical cord sera for the treatment of severe dry eye disease: a double-blind randomized clinical trial. Acta Ophthalmologica. 2022;100(2).
4. Celebi ARC, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):619-26.
5. Yoon KC, Im SK, Park YG, Jung YD, Yang SY, Choi J. Application of Umbilical Cord Serum Eyedrops for the Treatment of Dry Eye Syndrome. Cornea. 2006;25(3):268-72.
6. Allam I. Autologous serum eye drops versus lacrimal gland injection of platelet-rich plasma for severe dry eye. Delta J Ophthalmol. 2021;22(4):251.
7. Noble BA. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. British Journal of Ophthalmology. 2004;88(5):647-52.
8. Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial Effect of Artificial Tears Made with Autologous Serum in Patients with Keratoconjunctivitis Sicca. Arthritis & Rheumatism. 1984;27(4):459-61.
9. Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the Efficacy of 50% Autologous Serum Eye Drops in Different Ocular Surface Pathologies. BioMed Research International. 2014;2014:1-11.
10. Lee GA, Chen SX. Autologous serum in the management of recalcitrant dry eye syndrome. Clin Exp Ophthalmol. 2008;36(2):119-22.
11. Hwang J, Chung SH, Jeon S, Kwok SK, Park SH, Kim MS. Comparison of Clinical Efficacies of Autologous Serum Eye Drops in Patients With Primary and Secondary Sjögren Syndrome. Cornea. julho de 2014;33(7):663-7.
12. Cho YK, Huang W, Kim GY, Lim BS. Comparison of Autologous Serum Eye Drops with Different Diluents. Current Eye Research. 2013;38(1):9-17.
13. Avila MY, Igua AM, Mora AM. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. Br J Ophthalmol. 2019;103(5):648-53.
14. Ribeiro MMR, Timbó F, Ribeiro E, Ribeiro LE. The long-term effects of platelet -rich plasma in diabetic dry eye: a series of cases. Revista Brasileira de Oftalmologia. 2019;78(1).
15. Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. Cochrane Eyes and Vision Group, organizador. Cochrane Database of Systematic Reviews. 2017; 2017(2).
16. Ribeiro MVMR, Barbosa FT, Ribeiro LEF, Lacet CMC, Lyra JM de AG, Guedes V de L, et al. Platelet-rich plasma in diabetic dry eye disease. Revista Brasileira de Oftalmologia. 2016;75.
17. Mendes KDS, Silveira RC de CP, Galvão CM. Revisão integrativa: método de pesquisa para a incorporação de evidências na saúde e na enfermagem. Texto contexto - enferm. 2008;17(4):758-64.
18. Jirsova K, Brejchova K, Krabcova I, Filipec M, Al Fakih A, Palos M, et al. The Application of Autologous Serum Eye Drops in Severe Dry Eye Patients; Subjective and Objective Parameters Before and After Treatment. Current Eye Research. 2014;39(1):21-30.
19. Van der Meer PF, Verbakel SK, Honohan Á, Lorinser J, Thurlings RM, Jacobs JFM, et al. Allogeneic and autologous serum eye drops: a pilot double-blind randomized crossover trial. Acta Ophthalmologica. 2021;99(8):837-42.
20. García-Conca V, Abad-Collado M, Hueso-Abancens JR, Mengual-Verdú E, Piñero DP, Aguirre-Balsalobre F, et al. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. 2019;97(2).
21. Metheetrairut C, Ngowyutagon P, Tunganuntarat A, Khowawisetsut L, Kittisares K, Prabhasawat P. Comparison of epitheliotrophic factors in platelet-rich plasma versus autologous serum and their treatment efficacy in dry eye disease. Sci Rep. 2022;12(1):8906.
22. Avila MY. Restoration of Human Lacrimal Function Following Platelet-Rich Plasma Injection. Cornea. 2014;33(1):18-21.
23. Semeraro F, Forbice E, Nascimbeni G, Taglietti M, Romano V, Guerra G, et al. Effect of Autologous Serum Eye Drops in Patients with Sjögren Syndrome-related Dry Eye: Clinical and In Vivo Confocal Microscopy Evaluation of the Ocular Surface. 2016;30(6):931-8.
24. Panda A, Jain M, Vanathi M, Velpandian T, Khokhar S, Dada T. Topical Autologous Platelet-Rich Plasma Eyedrops for Acute Corneal Chemical Injury. Cornea. 2012;31(9):989-93.
25. Tobita M, Tajima S, Mizuno H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res Ther. 2015;6(1):215.
26. Mohammed MA, Allam IY, Shaheen MS, Lazreg S, Doheim MF. Lacrimal gland injection of platelet rich plasma for treatment of severe dry eye: a comparative clinical study. BMC Ophthalmol. 2022;22(1):343.
27. Kawakita T. Regeneration of Lacrimal Gland Function to Maintain the Health of the Ocular Surface. Invest Ophthalmol Vis Sci. 2018;59(14):DES169.
AUTHORS INFORMATIONS |
|
 |
» Vitor Souza Magalhães https://orcid.org/0000-0003-4308-4390 https://lattes.cnpq.br/8263955563980494 |
 |
» Milena Souza Ribeiro Santos https://orcid.org/0000-0001-5559-5986 https://lattes.cnpq.br/2572273146669592 |
 |
» Marina Viegas Moura Rezende Ribeiro https://orcid.org/0000-0001-7626-2806 https://lattes.cnpq.br/1596177314440842 |
Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
May 19, 2023.
Accepted on:
June 14, 2024.