José Maurício Botto Garcia1; Tainara Sardeiro de Santana1; David Leonardo Cruvinel Isaac2; Marcos Pereira Ávila2
DOI: 10.17545/eOftalmo/2021.0030
ABSTRACT
Myopia is the most common ocular disorder worldwide, and its incidence is increasing rapidly. The term “high myopia” refers to a higher axial length (typically over 26.50 mm) and a spherical equivalent higher than -6.0D. In 1970, myopic maculopathy had its first definition reported, including the presence of chorioretinal atrophy; central pigment spots; lacquer cracks; posterior staphyloma; and optic disc changes. The purpose of the authors is to depict the status of optical coherence tomography B-scans helping retina specialists on diagnosis and therapeutic decision on macular traction maculopathy. Individuals with high myopia can suffer from the development of several macular pathologies, such as myopic foveoschisis, macular hole with and without retinal detachment. Recent advances in imaging techniques, our understanding of macular pathology in myopia has significantly improved. New conditions have been identified and defined. Our purpose is to highlight the importance of optical coherence tomography when monitoring patients, using recently published data, in order to achieve the best possible results.
Keywords: Myopia; Tomography optical; Macular.
RESUMO
A miopia é o distúrbio ocular mais comum em todo o mundo e sua incidência está aumentando rapidamente. O termo “alta miopia” refere-se a um maior comprimento axial (tipicamente acima de 26,50 mm) e um equivalente esférico maior que −6,0 D. Em 1970, foi elaborada a primeira definição da maculopatia miópica, que incluía a presença de atrofia coriorretiniana, manchas pigmentares centrais, fissuras craqueladas, estafiloma posterior e alterações do disco óptico. O objetivo dos autores é descrever o status atual da tomografia de coerência óptica em B-scans, ajudando especialistas em retina no diagnóstico e decisão terapêutica sobre a maculopatia por tração macular. Indivíduos com alta miopia podem desenvolver várias patologias maculares, como foveosquise miópica e orifício macular, com ou sem descolamento de retina. Após avanços recentes nas técnicas de imagem, nossa compreensão da patologia macular na miopia melhorou significativamente. Novas condições foram identificadas e definidas. Os autores desejam destacar a importância da tomografia de coerência óptica no monitoramento de pacientes, usando dados publicados recentemente, a fim de alcançar os melhores resultados possíveis.
Palavras-chave: Miopia; Tomografia óptica; Macular.
INTRODUCTION
Myopia is the most common ocular disorder worldwide, and its incidence is increasing rapidly. In 2010, up to 27% of the world’s population was myopic, and 2.8% had high myopia. These numbers are projected to rise to 52% and 10%, respectively, by 2050. The term “high myopia” refers to a higher axial length (typically over 26.50 mm) and a spherical equivalent (SphE) higher than -6.0D1,2. In 1970, myopic maculopathy had its first definition reported, including the presence of chorioretinal atrophy; central pigment spots; lacquer cracks; posterior staphyloma; and optic disc changes. In 1984, Avila et al firstly proposed a classification system to describe the natural course of macular atrophy secondary to myopic maculopathy based on fluorescein angiography and stereoscopic fundus photographs3.
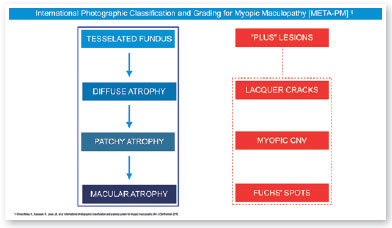
In 2015, an international panel recommended a novel photographic grading system for myopic maculopathy called the META-PM classification. This study published long-term data regarding natural progression of myopic maculopathy, describing main signs in categories: no myopic changes (category 0), tessellated fundus (category 1), diffuse chorioretinal atrophy (category 2), patchy chorioretinal atrophy (category 3), and macular atrophy (category 4) (Figure 1). Lesions that develop independently from the main progress pattern were considered “plus lesions,” and included lacquer cracks, myopic choroidal neovascularization (MCNV), and Fuchs’ spot (scar phase of myopic CNV) (Figure 2). Patients with lesions more severe than or equal to category 2 or with “plus lesions” were applied to the term “pathologic myopia”4. Still, META-PM presented limitations, such as the fact that fundus imaging may look different according to the degree of fundus pigmentation among races, and some macular conditions, such as myopic traction maculopathy (MTM) (Figure 2), and dome-shaped macula (DSM) (Figure 4) were not included5.
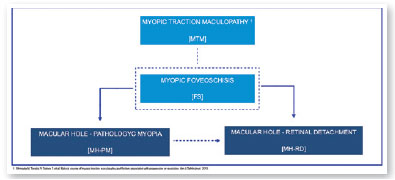
Current concept of MTM comprises a large spectrum of presentation that include Myopic Foveoschisis (FS), a persistent macular traction with intraretinal schisis, High Myopia Macular Hole (MH-HM) with and without retinal detachment (MH-RD). Its prevalence is reported to be ranging from 9% to 34.4% in highly myopic patients. Optical coherence tomography (OCT) is a non-invasive tool that allows detection and follow-up of many complications in high myopic patients, including posterior staphyloma (Figure 5), MTM, MH-HM, and MH-RD, performing an important role in such diseases.
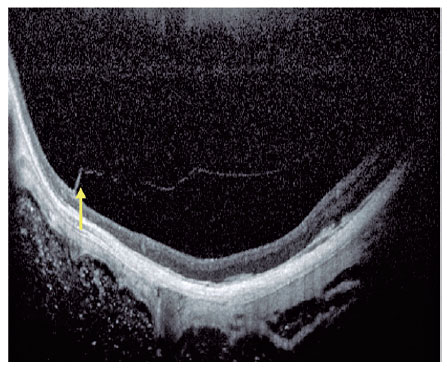
Several advances in imaging technology have enabled detailed in vivo study of highly myopic patients’ profile. Swept-source OCT (SS OCT) has been used to identify decreased choroidal thickness (CT) as an important feature in macular atrophy and has demonstrated that lower CT was more associated with myopic macular atrophy than scleral thickness6. OCT angiography portrayed that myopic CNV maintains detectable blood flow in the atrophic phase long after the onset of CNV. In the active phase, a clear vascular network is visible, but in the scar phase, the CNV is shrunken and irregularly shaped. In the atrophic phase, it is still possible to detect CNV with blood flow7.
The author’s objective in to demonstrate the importance of OCT in MTM, discussing optimal timing for surgery or clinical follow-up, to reach the best possible results., depicting how the status of OCT B-scans may help retina specialists on both diagnosis and therapeutic decision on MTM.
METHODS
This article presents a non-systematic literature review. For the survey of the literature, articles were searched in National Library of Medicine, comprised in MEDLINE dataset (PubMedTM). The descriptors used were myopic traction maculopathy (MTM), Myopic Foveoschisis (FS), High Myopia Macular Hole (MH-HM) with and without retinal detachment (MH-RD), and optical coherence tomography (OCT). For the selection of proper items, particularly relevant articles on the subject were included.
1. Foveoschisis and High Myopia:
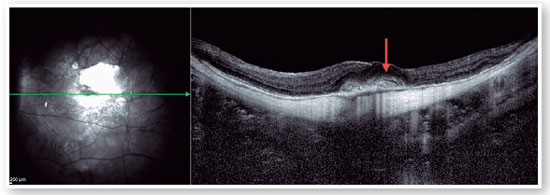
Myopic foveoschisis (FS) is a sight-threatening complication of pathological myopia or high myopia1,8,9. FS is the splitting of the retinal layers in the macula, causing macular accumulation of intraretinal and subretinal fluid in the absence of a full-thickness MH with intraretinal cysts or intraretinal columns (Figure 6)9-11. It is thought that incomplete or anomalous posterior vitreous detachment (PVD) is related to the pathogenesis of FS, and contraction of cortical vitreous can induce this intraretinal splitting9,11. Several complications may occur during the progression of FS, such as MH-HM and MH-RH, that may lead to visual loss. Its prevalence ranges from 9 to 20% of highly myopic populations1,12-16. Therefore, the clinical importance of FS is not negligible.
Posterior staphyloma is a posterior bulging or ectasia of the globe, that might be present in patients with high myopia. It is currently understood that patients with posterior staphyloma, allied with higher axial length, develop higher risk of developing FS8,17-19. The pathogenesis of FS includes abnormal traction by the posterior hyaloid, combined with anatomic changes in the vitreomacular interface triggered by the abnormal contour of the posterior staphyloma8. Ohno Matsui’s group classified this disease into five different categories based on location and the size of the affected area, ranging from no apparent foveoschisis (S0) to complete macular involvement (S4) (Figure 7).
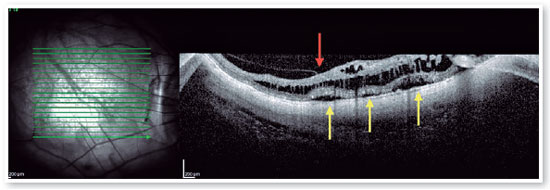
Pars plana vitrectomy with ILM peeling (with or without gas tamponade) is the mainstay for surgical treatment of FS5,8. The natural history of FS is generally poorly understood. Spectral-domain OCT (SD-OCT) is extremely useful in the assessment of FS. SD-OCT B-scans can show splitting of the neurosensory retina and subsequent epiretinal membrane associated with vitreomacular traction20. The backstory is about deciding to indicate surgical approach or follow patients, based on OCT findings. FS is thought to progress to lamellar MH and or foveal detachment (FD).
Optimal timing for surgery in patients with FS remains controversial, but SD-OCT specific features may help us to decide if vitrectomy is the most appropriate option for the patients. Gaucher et al retrospectively reviewed 29 charts of eyes during up to 31.2 months. Visual acuity worsened in 20 (69%) eyes and was stable in 9 (31%) eyes. In 9 of the 29 eyes, MH-HM developed during the follow-up period; 6 of the 9 eyes that developed MH-HM had foveal detachment prior to MH formation. Gaucher et al reported that MTM with FD was likely to develop a full thickness MH19. In an observational case series regarding surgical outcomes for MTM, MH-HM, and MH-RD, the mean BCVA at 6 months after vitrectomy in eyes treated for MH-RD was significantly worse compared with that of eyes treated for MTM without MH. Thus, it is preferable to operate before the development of MH-RD1,8,21,22.
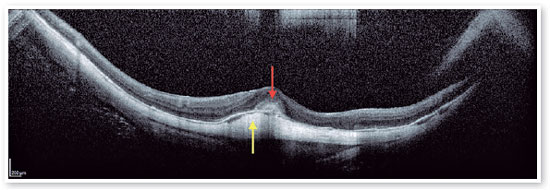
Therefore, patients with FS should be monitored regularly for foveal detachment, and surgical treatment should be considered when foveal detachment develops aiming to prevent the development of full-thickness macular hole (FTMH), and subsequent MH-RD21 (Figures 8 and 9). Eyes that also present with preoperative ellipsoid disruption and thinner central foveal thickness also tend to have poorer visual outcomes 21.
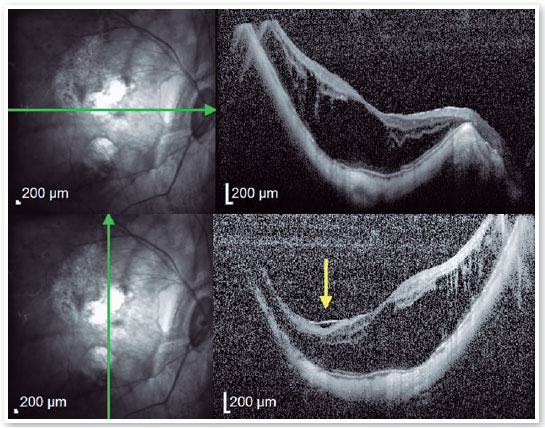
2. High Myopia Macular Hole (MH-HM):
MH-HM is a relatively common complication in MTM patients with an axial length larger than 26.5 mm and/or a refraction greater than −6.00 diopters1,21. Its prevalence is about 8.4%5. Macular hole is also recognized as a predisposing factor to posterior pole RD11,12,21,22. OCT B-scans depicting FS bring together a worst anatomic and functional prognosis, and its headways to a more advanced stage, as long as persistent traction remains acting, which may result in the formation of a MH-HM. Spontaneous resolution of MTM, including MH-HM without surgical approach is rare, but has been reported24. The development of a MH-HM is associated with anteroposterior and tangential traction over the macula, closely related to what occurs in emmetropic eyes. However, the manifestation of a posterior staphyloma, promoting retinal layer splitting, commonly links MH-HM with FS12,22. Shimada et al. reported that although 3.9% of MTMs developed RD, the decision about adopting a surgical intervention should be taken carefully. However, 27.3% of MH-HM occurred after surgery for MTM8,23.
It is difficult to determine whether surgical intervention is necessary and to determine the ideal timing of the surgery because exacerbation and natural improvement are both possible1,12. MH-HM with FS was reported to have a worse outcome than without FS8,23,24. Since the introduction of three-port pars plana vitrectomy with gas tamponade, several surgical strategies have been developed for MH-HM repair in high myopic patients, including silicone oil tamponade, both posterior and suprachoroidal macular buckling (MB), and scleral shortening technique1,12,14,15,21,25. Previous studies have shown that procedures that use heavy silicone oil have a reattachment rate of approximately 87%, compared with a reattachment rate of 53% for procedures using standard silicone oil. However, despite the higher success rate with heavy silicone oil, there was no statistically significant difference in final vision12. The technical difficulties inherent to MB, and risk of injury to the surrounding structures seemed to restrict this procedure from becoming common. But as time went on, MB has been gradually recognized as a major procedure to mechanically counteract the pulling effects of the posterior staphyloma, relaxing the retina, thus facilitating MH closure25.
The presence of FS indicates that the posterior staphyloma plays an important role in the pathogenesis retinal stretching by producing outward tension on the stiff inner vitreoretinal complex including a rigid ILM. Consequently, relieving both anteroposterior and tangential traction over the macula by vitrectomy combined with ILM removal may stretch the MH because the retina would be forced to attach to the wider arc of the deep posterior scleral wall (Figure 10)9,12,19,21. MH-HM without RD can be classified into two categories based on foveal structure: schisis type and flat type1. MB surgery may be beneficial for this type of MH-HM, but its invasive nature and possible compromised choroidal circulation might. Be considered. In contrast, MH-HM without FS has a pathogenesis similar to idiopathic MHs and has more favorable postoperative outcomes25.
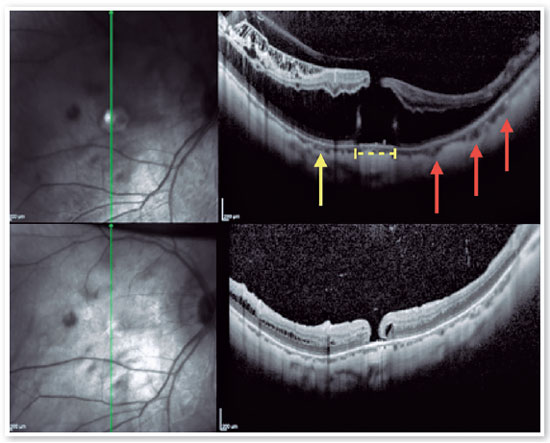
3. Macular Hole - Retinal Detachment (MH-RD):
MH-RD is one of the most threatening complications to high myopic eyes, and it is reported in about 67.7 % of them (Figures 11 and 12)26,27. Although the pathogenesis is not completely understood higher axial length, posterior staphyloma formation, tangential traction of vitreous cortex, rigidity of ILM and epiretinal membrane may be related factors2. For this reason, vitrectomy combined with ILM peeling was believed to be one of the beneficial procedures for those patients. Rationale includes relieving macular traction completely and increasing flexibility of the retina to conform better to the posterior staphyloma21,26,27. Intraoperative OCT has been reported to affect the surgical decision in more than 40% cases, helping in identification of ILM/epiretinal membrane (ERM) cleavage sites, and playing a significant role in decision making22,26.
Although MH-RD are usually treated with vitreous surgery to release vitreous traction or with a gas tamponade, their prognosis is poor27.
Surgical approach for MH-RD is slightly different than surgery for MH-HM without concurrent RD, and frequently more challenging. MH-RD is a refractory disease with poor visual prognosis. Despite all potential and promising interventions, macular hole reopening and recurrent retinal detachment may still develop, mainly for extreme high axial myopia2,15,23,27. Inverted ILM flap technique to treat MH-RD has been presented satisfactory results. The anatomic success of inverted ILM flap for myopic MH without retinal detachment ranged from 94 to 100%, but a 100% closure rate has been reported in patients with MH-RD14,16,26,27.
4. Surgical Myopic Traction Maculopathy (MTM):
Primary challenge of surgical treatment of MTM is the peeling the ILM over the detached and mobile retina. ILM peeling is crucial in MH-RD (au contraire from MH without RD) in order to release all tractional forces. Perfluorcarbon liquid (PFCL) assisted technique may help to keep the retina firm and less mobile during ILM peeling. Furthermore, it provides a better staining prior the peeling itself. Small amount of PFCL is injected into the eye to cover the macular hole. Diluted indocyanine green (ICG) or brillhant blue G (BBG) is injected around the PFCL bubble to stain the ILM. The PFCL prevents the dye from gaining access to the subretinal space. Getting a good stain is critical for a complete ILM peeling. By tilting the eye, one can steamroll the dye under the PFCL and over the ILM to facilitate a good stain14,16,28,29.
To prevent complications resulting from iatrogenic trauma, long-shaft forceps for ILM peeling specific for highly myopic eyes were developed. 27+ forceps have been developed with a distended grasping platform, with the area almost equal to that of the 25+ gauge forceps. It is 59% larger than the prior 27-gauge platform, and therefore proportionately reduces pressure on the membrane and minimizes the likelihood of it being shredded by excessive pressure. The 27+ forceps features the same conformal grasping platform as the 23 and 25+ gauge versions, with a more rectangular shape. This may reduce the risk of pinching the retinal nerve fiber layer with the edge of the grasping platform when grasping in an angled configuration. Regarding ILM technique, pinch-and-peel may work best to initiate peeling in a detached and mobile retina12,24,28,29.
Macular buckling (MB) surgery is an alternative approach to treat recurrent or difficult cases of MH-RD. It helps MH closure by inserting a ridge that pushes the whole macula forward. This could explain why myopic eyes with MH and dome shaped macula (DSM) are less susceptible to RD; as occurs in eyes with implanted MB, DSM reduces the curvature of the staphyloma and may prevent retinal detachment19,25. In this regard, some authors have reported better results with MB than with vitrectomy, even in primary MH-RD. Recent reports suggest that combining MB and vitrectomy with ILM peeling may be a promising alternative in refractory cases25,29.
Individuals with high myopia may suffer from the development of several macular pathologies such as myopic FS, MH-HM, and MH-RD. With the recent advances in imaging techniques, such as SD-OCT and SS-OCT, our understanding of macular pathology in myopia has significantly improved. New conditions such as MTM have been identified and defined. The optimal timing for surgery in patients with FS remains controversial, but SD-OCT specific features may help us to decide if vitrectomy is the most appropriate option for our patients. Therefore, patients with FS should be monitored regularly for foveal detachment, and surgical treatment should be considered when foveal detachment develops. It is reported occasionally rapid development of MTM from severe FS to foveal detachment or micro-MH, even when the waiting period for surgery was short as advised after careful deliberation based on OCT B-scans imaging. Treatment approaches are now being planned on the basis of the pathoanatomic features of MTM on OCT. It is proposed that the best time to perform vitrectomy for FS is when visual acuity just starts to worse, before the appearance of foveal detachment, or at the moment of its diagnosis. It’s important to highlight the importance of OCT during follow-up of patients diagnosed with MTM, aiming to achieve the best possible outcomes. Thus, OCT evaluation after surgery is also recommended.
REFERENCES
1. Gómez-Resa M, Burés-Jelstrup A, Mateo C. Myopic traction maculopathy. Dev Ophthalmol. 2014;54:204-12.
2. Hashimoto S, Yasuda M, Fujiwara K, Ueda E, Hata J, Hirakawa Y, et al. Association between Axial Length and Myopic Maculopathy: The Hisayama Study. Ophthalmol Retina. 2019;3(10):867-73.
3. Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91(12):1573-81.
4. Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CMG, Saw SM, Verhoeven VJM, Klaver CCW, Moriyama M, Shinohara K, Kawasaki Y, Yamazaki M, Meuer S, Ishibashi T, Yasuda M, Yamashita H, Sugano A, Wang JJ, Mitchell P, Wong TY, META-analysis for Pathologic Myopia (META-PM) Study Group. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877-83.e7.
5. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019 Mar;69:80-115.
6. Wong CW, Teo YCK, Tsai STA, Ting SWD, Yeo YSI, Wong WKD, et al. Characterization of the choroidal vasculature in myopic maculopathy with optical coherence tomographic angiography. Retina. 2019;39(9):1742-50.
7. Ishida T, Watanabe T, Yokoi T, Shinohara K, Ohno-Matsui K. Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br J Ophthalmol. 2019;103(4):457-62.
8. Shimada N, Ohno-Matsui K, Baba T, Futagami S, Tokoro T, Mochizuki M. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142(3):497-500.
9. Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond). 2013;27 Suppl 1(Suppl 1):S1-21.
10. Brockmann T, Steger C, Weger M, Wedrich A, Haas A. Risk assessment of idiopathic macular holes undergoing vitrectomy with dye-assisted internal limiting membrane peeling. Retina. 2013;33(6):1132-6.
11. Casuso LA, Scott IU, Flynn HW Jr., Gass JD, Smiddy WE, Lewis ML, et al. Long-term follow-up of unoperated macular holes. Ophthalmology. 2001;108(6):1150-5.
12. De Giacinto C, Pastore MR, Cirigliano G, Tognetto D. Macular Hole in Myopic Eyes: A Narrative Review of the Current Surgical Techniques. J Ophthalmol. 2019 Mar 11;2019:3230695.
13. Madi HA, Masri I, Steel DH. Optimal management of idiopathic macular holes. Clin Ophthalmol. 2016 Jan 13;10:97-116.
14. Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014; 34(4):664-9.
15. Morescalchi F, Costagliola C, Gambicorti E, Duse S, Romano MR, Semeraro F. Controversies over the role of internal limiting membrane peeling during vitrectomy in macular hole surgery. Surv Ophthalmol. 2017;62(1):58-69.
16. Oleñik A, Rios J, Mateo C. Inverted internal limiting membrane flap technique for macular holes in high myopia with axial length ≥30 mm. Retina. 2016;36(9):1688-93.
17. Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. Am J Ophthalmol. 1971;71(1 Pt 1):42-53.
18. Dai F, Li S, Wang Y, li S, Han J, Li M, et al. Correlation between posterior staphyloma and dome-shaped macula in high myopic eyes. Retina. 2020;40(11):2119-26.
19. Gaucher D, Erginay A, Lecleire-Collet A, Haouchine B, Puech M, Cohen SY, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol. 2008;145(5):909-14.
20. Fang Y, Du R, Nagaoka N, Yokoi T, Shinohara K, Xu X, et al. OCT-Based Diagnostic Criteria for Different Stages of Myopic Maculopathy. Ophthalmology. 2019;126(7):1018-32.
21. Lim LS, Ng WY, Wong D, Wong E, Yeo I, Ang CL, et al. Prognostic factor analysis of vitrectomy for myopic foveoschisis. Br J Ophthalmol. 2015;99(12):1639-43.
22. Gohil R, Sivaprasad S, Han LT, Mathew R, Kiousis G, Yang Y. Myopic foveoschisis: a clinical review. Eye (Lond). 2015;29(5):593-601.
23. Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156(5):948-57.e1.
24. Ono T, Terada Y, Mori Y, Kataoka Y, Nakahara M, Miyata K. Spontaneous resolution of myopic foveoschisis and a macular hole with retinal detachment. Am J Ophthalmol Case Rep. 2019 Jan 9;13:143-6.
25. Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):863-77.
26. Gao X, Guo J, Meng X, Wang J, Peng X, Ikuno Y. A meta-analysis of vitrectomy with or without internal limiting membrane peeling for macular hole retinal detachment in the highly myopic eyes. BMC Ophthalmol. 2016 Jun 13;16:87.
27. Yuan J, Zhang LL, Lu YJ, Han MY, Yu AH, Cai XJ. Vitrectomy with internal limiting membrane peeling versus inverted internal limiting membrane flap technique for macular hole-induced retinal detachment: a systematic review of literature and meta-analysis. BMC Ophthalmol. 2017;17(1):219.
28. Andrew N, Chan WO, Tan M, Ebneter A, Gilhotra JS. Modification of the Inverted Internal Limiting Membrane Flap Technique for the Treatment of Chronic and Large Macular Holes. Retina. 2016;36(4):834-7.
29. Chen SN, Yang CM. Inverted Internal Limiting Membrane Insertion for Macular Hole-Associated Retinal Detachment in High Myopia. Am J Ophthalmol. 2016 Feb;162:99-106.e1.
AUTHOR’S INFORMATION

Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
November 13, 2020.
Accepted on:
October 14, 2021.