Filipe Moreira de Araújo1,2; Paula Paiva Pegoraro1,2; Fernando Paganelli1,2,3
DOI: 10.17545/eOftalmo/2021.0010
ABSTRACT
Keratoconus is rarely an emergency, unless when there is corneal hydrops, a rupture of Descemet’s membrane that allows aqueous outflux into the corneal stroma, resulting in abrupt vision reduction, and can be accompanied by pain, discomfort and photophobia. The treatment of this clinical condition has been controversial. This report presents a clinical case in which the patient with a diagnosis of keratoconus in both eyes attended the emergency service with pain, itching and photophobia in the right eye, with sudden onset. She was instructed to fast and rest and eye drops of the following solutions were prescribed: dimethylpolysiloxane four times a day, tropicamide three times a day and moxifloxacin every hour until the proposed intervention: intraestromal corneal injection of 100µL of autologous blood. In the immediate postoperative period, absence of Seidel or hyphema was noted. During the follow-up, the patient remained asymptomatic with notable autologous blood resorption, awaiting penetrating corneal transplant. Filling stromal clefts with autologous blood proved to be effective in blocking aqueous leakage by a process of corneal perifistular coagulation/inflammation. Therefore, a safe method of “damage containment” until the viability of the resolutive procedure is demonstrated.
Keywords: Cornea; Keratoconus; Ectasia; Complication; Hydrops.
RESUMO
Ceratocone raramente é uma emergência, a menos quando há hidropsia corneana, ruptura da membrana de Descemet que permite o vazamento do humor aquoso para o estroma corneano, levando à redução abrupta da visão, podendo ser acompanhada de dor, desconforto e fotofobia. O tratamento desta condição clínica tem sido controverso. Este relato apresenta um caso clínico em que a paciente com diagnóstico de ceratocone em ambos os olhos compareceu ao serviço de emergência com quadro de dor, irritação e fotofobia no olho direito, com início súbito. Orientou-se jejum e repouso e foram prescritos colírios das seguintes soluções: dimetilpolisiloxane quatro vezes ao dia, tropicamida três vezes ao dia e moxifloxacino a cada hora até a intervenção proposta: injeção intraestromal corneana de 100µL de sangue autólogo. No pós-operatório imediato notava-se ausência do sinal de Seidel ou hifema. Durante o seguimento, a paciente manteve-se assintomática com notável reabsorção do sangue autólogo, aguardando transplante penetrante de córnea. O preenchimento das fendas estromais com sangue autólogo mostrou-se eficaz em bloquear o vazamento do aquoso mediante processo de coagulação/inflamação perifistular corneana. Demonstra-se, portanto, um método seguro de “contenção de danos” até a viabilidade do procedimento resolutivo.
Palavras-chave: Ceratocone; Córnea; Ectasia; Complicação; Hidropsia.
INTRODUCTION
Anterior keratoconus is a noninflammatory corneal ectasia characterized by a thinning of the central or paracentral corneal stroma, accompanied by apical protrusion (cone shape) and irregular astigmatism1. It is usually bilateral, asymmetric, with greater progression in adolescence; however, when diagnostic and imaging criteria allow subclinical detection, unilateral involvement is noticed in up to 4% of cases2. Family history is present in only 6–8% of cases, suggesting autosomal dominant transmission with incomplete penetrance. The association with systemic diseases, such as atopic disease (whose relation to the anterior keratoconus occurs in up to 53% of the cases), Down syndrome or some noninflammatory conditions of connective tissue, such as Ehlers–Danlos and osteogenesis imperfecta3, is already well described in the literature4. These diseases must be related to keratoconus due to microtrauma caused by scratching the eyes1. The diagnosis is made by biomicroscopy, keratometry, keratoscopy, corneal topography and optical coherence tomography (OCT) of the anterior ocular segment4.
The treatment of keratoconus depends on the case status and ranges from general behavioral precautionary measures, progression control (corneal crosslinking) and visual rehabilitation (correction with glasses, fitting with hard contact lens, intrastromal ring, to corneal transplants, lamellar or not)1. It is rarely an emergency, unless there is corneal hydrops, a rare event in keratoconus (3%)5 defined by a rupture of Descemet’s membrane that allows sudden aqueous outflux into the corneal stroma leading to abrupt vision loss, which may be accompanied by pain, discomfort and photophobia6. The treatment proposed in the acute episode of hydrops is topical administration of cycloplegic and hypotensive eye drops, and hypertonic saline solution, use of compressive dressings and therapeutic contact lenses1.
The accelerated resolution of the acute phase with intracameral gas injection has been described in the literature, as it works as a mechanical barrier reducing the flow of aqueous humor into the stroma; gases that remain for a longer time in the anterior chamber, such as sulfur hexafluoride (SF6 at 20% remains for more than two weeks) and perfluorpropane (C3F8 at 14% remains for more than six weeks) are preferred7. Spontaneous healing of the rupture occurs from six to twelve weeks, usually with a significant worsening of vision due to stromal opaqueness, requiring penetrating keratoplasty if there is no improvement of the edema in three months or if there is a stromal scar that compromises the visual axis1.
The scenario of corneal hydrops with spontaneous perforation is very rare8,9 which becomes the treatment priority. Filling the stromal clefts, inherent to the physiopathology of hydrops10, with autologous blood has proven effective in blocking aqueous leakage by the process of perifistular corneal coagulation/inflammation. The objective of this report is to demonstrate an effective method in the management of hydrops in this condition.
CASE REPORT
Patient M. A. Q., a 24-year-old female, white and without systemic comorbidities, came to the emergency service with significant pain, itching and photophobia in the right eye (RE) for one day, with a sudden onset. She was doing ophthalmological follow-up due to the diagnosis of keratoconus in both eyes.
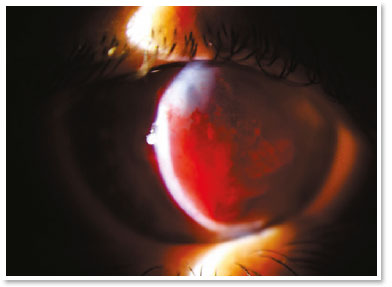
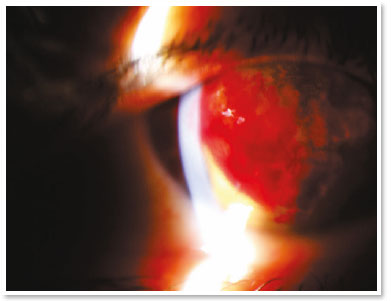
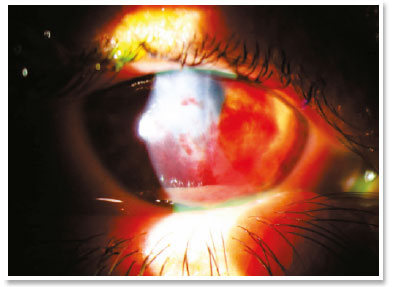
She presented visual acuity (VA) of hand motion in the RE and 0.4 in the left eye (LE). Biomicroscopy of the RE showed central corneal hydrops of approximately 8 × 7mm with superior temporal active Seidel; in the LE, only Fleischer ring was noteworthy. Alternatives such as contact lens or amniotic membrane coating to treat the perforation were not feasible at the time (impossible lens adaptation due to the pronounced protuberance of hydrops). Fasting and resting were oriented and eye drops of the following solutions were prescribed: dimethylpolysiloxane four times a day, tropicamide three times a day and moxifloxacin every hour until the proposed intervention.
In the surgical center, under topical anesthesia (tetracaine hydrochloride 1%/phenylephrine hydrochloride 0.1%) and sterile conditions after periocular application of povidone-iodine 10% in the RE and placement of surgical fields, 3ml of peripheral venous blood was aspirated from right upper limbs via previous puncture. Approximately 100μl of this autologous blood were injected into the corneal stroma with an insulin syringe and a 30-gauge needle (manually angled at approximately 45 degrees to technically facilitate continuous injection under a surgical microscope). The needle bevel was directed to the corneal stroma near the stromal cyst filled with aqueous humor in order to observe the blood taking the space of the intracorneal aqueous humor. The evaluation of the anterior segment a few moments after injection also certified the absence of blood in the anterior chamber.
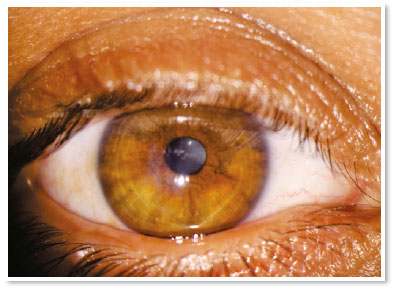
In the immediate postoperative period, there was no aqueous leakage or hyphema, and moxifloxacin eye drops were maintained until the first postoperative day (when it was suspended). During the entire follow-up up to the sixth postoperative month, the patient remained asymptomatic and with remarkable resorption of autologous blood, waiting for penetrating corneal transplant of the RE.
DISCUSSION
The scenario of a corneal hydrops deserves special attention in terms of management, given the sudden worsening of visual acuity, discomfort and important photophobia. The understanding of its pathophysiology is already a fundamental reality to guide a therapeutic approach. The rupture of Descemet’s membrane, which allows aqueous humor to enter the stroma, causes the membrane to retract when ruptured under pressure, so that the perfect realignment of the two parts is not possible either spontaneously or with some type of treatment11. Regression of hydrops therefore occurs in two steps: first, reattachment of Descemet’s membrane to the posterior stroma and, second, migration of endothelial cells to the space between membrane’s parts. The time to resolution as well as the prognosis is related to the rupture size6.
The treatment with intracameral gas (C3F8 or SF6) aims to accelerate the first step, but there is much discussion about the validity of this injection in the face of some facts, such as the need for reapplications and prolonged dorsal decubitus. It is known that corneal hydrops is self-limited in approximately four months and studies have shown that there is no significant difference in its use with respect to final visual acuity or density of endothelial cells12. Uncommon but severe events such as pupillary block or Urrets-Zavalia syndrome should be kept in mind13. On the other hand, accelerating the attachment of Descemet’s membrane seems to reduce the stimulus to develop corneal neovascularization (something crucial in terms of prognosis for a future transplant)11. The choice for gas in this case of hydrops with positive Seidel’s sign did not seem to be a good option in the face of a possible intrastromal migration of the gas and, even, the need to adhere to the supine position for a long period (two weeks) of a patient in labor needs.
Alió et al.14 described a successful management of a case of corneal OCT-guided hydrops with hemoderivative autologous platelet-rich plasma in a patient with Down syndrome. In this case, however, intracameral injection was performed. The idea of using hemoderivative is justified by its high level of growth factors, cytokines and cell adhesion molecules essential in the healing process. Much has recently been produced about the use of so-called “eye platelet-rich plasma” in dry eye syndrome, ocular surface reconstruction and persistent corneal ulcers. It is obtained using 3.2% sodium nitrate as an anticoagulant and centrifugation to separate the plasma, which is rich in platelets15.
The particularity of the present case of hydrops with positive Seidel’s sign adds to the objective of the treatment not only means to restore the stromal architecture, but also a way to seal the intrastromal duct by which the aqueous humor leaked out of the eye. Autologous blood injected into the stroma was able to stop the leak after intrastromal coagulation, in addition to carrying with it the benefits of the hemoderivative. Since the injection is intraestromal, it reduces the possibility of elevation, even transitory, of the intraocular pressure, as noted in the case described by Alió et al14.
The use of amniotic membrane in ocular surface reconstruction is already well established and it acts facilitating epithelial migration, reinforcing basal cell adhesion, promoting epithelial differentiation and preventing apoptosis16. Its use in hydrops with positive Seidel’s sign would therefore function more superficially, so that the intrastromal path followed by the aqueous humor would continue existing. Azuara-Blanco et al.17 also demonstrated in a ten-case study the inefficiency of using amniotic membrane as a patch in impending or recent perforation.
Similarly, cyanoacrylate did not seem to be a good alternative to the case, since it is not biodegradable and induces inflammatory response, corneal neovascularization, foreign body sensation and tissue necrosis18. Even fibrin glue, which does not have such disadvantages of cyanoacrylate, since it is a biological and biocompatible compound19, had not been considered for the case because its mechanism of action to contain the aqueous humor leak would only act on the external surface of the cornea, despite the proposed intraestromal autologous blood that would be able to block the entire aqueous humor route through the cornea to external environment. In addition, fibrin glue is more expensive to the process. The use of therapeutic contact lens was anatomically unfeasible to that corneal anatomy.
The lesion located on the visual axis is a prediction of scar formation with notable influence on the visual prognosis, with the need for corneal transplantation for optical treatment. However, penetrating transplant was not an option considered, as high rejection rates have already been determined in acute settings20.
The use of intraestromal autologous blood has proved to be an effective alternative: its coagulation in the clefts inherent to the pathogenesis of hydrops would prevent both stromal drenching by aqueous humor and its leakage with the benefits of a hemoderivative15, while the intracameral infusion already described as successful by Alió et al.14 had not been suggested in this case, by aspects such as peak pressure due to the difficulty of draining the aqueous humor by deposition of human plasma proteins in the trabecular meshwork, simulating, for example, a previous uveitis21.
The filling of stromal clefts, inherent to the pathophysiology of hydrops, with autologous blood has proven effective in blocking aqueous leakage by the process of corneal perifistular coagulation/inflammation. Thus, a safe method of “damage containment” to the viability of the resolutive procedure (penetrating transplant) is demonstrated.
REFERENCES
1. Mannis MJ, Holland EJ. Cornea: Fundamentals, diagnosis and management. Amsterdam: Elsevier; 2017.
2. Rabinowitz YS, Nesburn AB, McDonnell PJ. Videokeratography of the fellow eye in unilateral keratoconus. Ophthalmology. 1993;100(2):181-186. doi: 10.1016/S0161-6420(93)31673-8
3. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319. doi: 10.1016/S0039-6257(97)00119-7
4. Wagner H, Barr JT, Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: Methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-232. doi: 10.1016/j.clae. 2007.03.001
5. Mohebbi M, Pilafkan H, Nabavi A, Mirghorbani M, Naderan M. Treatment of acute corneal hydrops with combined intracameral gas and approximation sutures in patients with corneal ectasia. Cornea. 2020;39(2):258-262. doi: 10.1097/ICO.0000000000002155
6. Basu S, Vaddavalli PK, Vemuganti GK, Hasnat A, Murthy SI. Anterior segment optical coherence tomography features of acute corneal hydrops. Cornea. 2012;31(5):479-485. doi: 10.1097/ICO.0b013e318223988e
7. Panda A, Aggarwal A, Madhavi P, et al. Management of acute corneal hydrops secondary to keratoconus with intracameral injection of sulfur hexafluoride (SF6). Cornea. 2007;26(9):1067-1069. doi: 10.1097/ICO.0b013e31805444ba
8. Rubsamen PE, McLeish WM. Keratoconus with acute hydrops and perforation. Brief case report. Cornea. 1991;10(1):83-84. doi: 10.1097/00003226-199101000-00019
9. Nicoli C, Wainsztein RD, Trotta LP. Corneal topography of spontaneous perforation of acute hydrops in keratoconus. J Cataract Refract Surg. 1999;25(6):871-872. doi: 10.1016/S0886-3350(99)00028-0
10. Greenwald MF, Vislisel JM, Goins KM. Acute corneal hydrops [Internet]. EyeRounds.org; 2016. [cited 2020 5 20]. Available at: http://eyerounds.org/cases/241-acute-corneal-hydrops.htm
11. Fan Gaskin JC, Patel DV, McGhee CNJ. Acute corneal hydrops in keratoconus-new perspectives. Am J Ophthalmol. 2014;157(5): 921-928. doi: 10.1016/j.ajo.2014.01.017
12. Basu S, Vaddavalli PK, Ramappa M, Shah S, Murthy SI, Sangwan VS. Intracameral perfluoropropane gas in the treatment of acute corneal hydrops. Ophthalmology. 2011;118(5):934-939. doi: 10.1016/j.ophtha.2010.09.030
13. Vanathi M, Mohan S, Dada T, Panda A. Malignant glaucoma after intracameral isoexpansile perfluoropropane tamponade for the management of acute corneal hydrops. Cornea. 2010;29(7):838 doi: 10.1097/ICO.0b013e3181c6b4e1
14. Alió JL, Toprak I, Rodriguez AE. Treatment of severe keratoconus hydrops with intracameral platelet-rich plasma injection. Cornea. 2019;38(12):1595-1598. doi: 10.1097/ICO.0000000000002070
15. Alió JL, Rodriguez AE, WróbelDudzińska D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol. 2015;26(4):325-332. doi: 10.1097/ICU.0000000000000169
16. Yildiz EH, Nurozler AB, Aksoy NO, Altiparmak UE, Onat M, Karaguzel H, et al. Amniotic membrane transplantation: indications and results. Eur J Ophthalmol. 2008;18(5):685-690. doi: 10.1177/ 112067210801800504
17. Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83(4):399-402. doi: 10.1136/bjo.83.4.399
18. Sharma A, Kaur R, Kumar S, Gupta P, Pandav, S, Patnaik B, et al. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003;110(2):291-298. doi: 10.1016/S0161-6420(02)01558-0
19. Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72(3):133-43. doi: 10.1046/j.1423-0410.1997.7230133.x. PMID: 9145483.
20. Bhatt P, Lim LT, Ramaesh K. Therapeutic deep lamellar keratoplasty for corneal perforations. Eye. 2007;21(9):1168-1173. doi: 10.1038/sj.eye.6702428
21. Epstein DL, Hashimoto JM, Grant WM. Serum obstruction of aqueous outflow in enucleated eyes. Am J Ophthalmol. 1978; 86(1):101-105. doi: 10.1016/0002-9394(78)90023-5
AUTHOR’S INFORMATION

Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
October 13, 2020.
Accepted on:
November 2, 2020.