Maurício B. Pereira1; João Paulo Lomelino2; Eduardo de França Damasceno3; Raul Nunes Galvarro Vianna4
DOI: 10.17545/eoftalmo/2019.0012
ABSTRACT
Retinal arterial macroaneurysms (RAMs) are commonly observed in the posterior pole, usually in the first arterial bifurcations, up to thirdorder vessels or arteriovenous crossings. Most RAMs are asymptomatic, with loss of vision occurring owing to hemorrhages or exudation with macular involvement. In general, RAM occurs in individuals aged 60–80 years, especially in women. Systemic arterial hypertension, age over 60 years, and the female sex are the main associated risk factors. The diagnosis is made based on ophthalmoscopy findings. Fluorescein angiography, indocyanine green angiography, and optical coherence tomography contribute to confirm the diagnosis. In this review article, we have described the epidemiology, pathogenesis, clinical presentation, findings of additional exams, differential diagnosis, systemic associations, pathology, treatment, and prognosis of RAM.
Keywords: Aneurysm; Retina; Hypertension; Eye Manifestations.
RESUMO
Os macroaneurismas arteriais retinianos (MAR) são comumente observados no pólo posterior, em geral nas primeiras bifurcações arteriais, até ramos de terceira ordem ou cruzamentos arteriovenosos. A maioria dos MAR são assintomáticos e a perda visual decorre de hemorragias ou exsudação com envolvimento macular. Geralmente, os MAR ocorrem em indivíduos entre 60 e 80 anos, preferencialmente do sexo feminino. A hipertensão arterial sistêmica, idade acima de 60 anos e o sexo feminino são os principais fatores de risco associados. O diagnóstico é feito pelos achados oftalmoscópicos. A angiografia fluoresceínica, a angiografia com indocianina verde e a tomografia de coerência óptica contribuem para confirmação diagnóstica. Neste artigo de revisão descreveremos a epidemiologia e patogênese, o quadro clínico, os achados de exames complementares, diagnóstico diferencial, associações sistêmicas, patologia, assim como tratamento e prognóstico.
Palavras-chave: Aneurisma; Retina; Hipertensão; Manifestações Oculares.
RESUMEN
Los macroaneurismas arteriales retinianos (MAR) son comúnmente observados en el polo posterior, en general en las primeras bifurcaciones arteriales, hasta ramas de tercera orden o cruces arteriovenosos. La mayoría de los MAR son asintomáticos y la pérdida visual adviene de hemorragias o exudación con comprometimiento macular. Generalmente, los MAR ocurren en individuos entre 60 y 80 años, preferencialmente del sexo femenino. La hipertensión arterial sistémica, la edad por encima de los 60 años y el sexo femenino son los principales factores de riesgo asociados. El diagnóstico se hace mediante hallazgos oftalmoscópicos. La angiografía fluoresceínica, la angiografía con indocianina verde y la tomografía de coherencia óptica aportan para confirmación diagnóstica. En este artículo de revisión, describiremos la epidemiología y la patogénesis, el cuadro clínico, los hallazgos de exámenes complementarios, diagnóstico diferencial, asociaciones sistémicas, patología, así como tratamiento y pronóstico.
Palabras-clave: Aneurisma; Retina; Hipertensión; Manifestaciones Oculares.
INTRODUCTION
Retinal arterial macroaneurysms (RAMs) are characterized by round or fusiform dilations of retinal arteries, which are usually unilateral and single and are located within the first arterial bifurcations, up to third-order branches or arteriovenous crossings at the posterior pole. Most RAMs are asymptomatic, and loss of vision occurs owing to hemorrhages or exudation with macular involvement1. Robertson described this condition in 19732 and named it retinal macroaneurysm owing to its size, which was larger than that of microaneurysms observed in diabetic retinopathy and retinal vein occlusion.
EPIDEMIOLOGY AND PATHOGENESIS
In general, RAM occurs in individuals aged 60–80 years, predominantly in females. Systemic arterial hypertension is commonly present (65%–75% patients) along with other risk factors for stroke and coronary heart diseases, such as arteriosclerosis and dyslipidemia3,4.
In a study conducted with 43 patients and 52 identified RAM paired with a similar control group, which aimed to identify ocular and systemic risk factors for RAM5, the findings confirmed that RAM is predominantly present in females, and there was a higher prevalence of arterial hypertension and retinal vein occlusion in group comprising patients than that comprising controls. The author observed a 12 times higher risk of RAM being located in an area affected by vein occlusion5. In another study, which analyzed 147 patients, an association between macroaneurysm and the area affected by retinal vein occlusion was observed; the following four categories of retinal macroaneurysms were determined: arterial, venous, capillary, and collateral-associated. A distinct characteristic of this RAM group was retinal neovascularization in 32% of the eyes6.
In a literature review, in which 60 patients with RAM were analyzed, RAM was predominantly present in females, reaching 80% of the sample. The mean age was 71 years, and systemic arterial hypertension was the most frequent associated finding (78% of cases)7.
Changes related to arterial hypertension and aging may explain the emergence of RAM, for example, substitution of the mu/scular middle layer of the arterial wall by collagen, hyaline degeneration of the vessel wall, endothelial lesion by atheromas with thickening of the vascular lumen, increased vascular stiffness, and increased intravascular and transmural pressure. The loss of local self-regulation and turbulence occurs later with dilation of focal artery and formation of the macroaneurysm7. Weakening of the arterial wall leads to focal dilation and formation of the aneurysm8,9.
There is histological evidence that local thromboembolic phenomena trigger the process that leads to the rupture of the macroaneurysm, with dissection of the inner vessel wall progressing to complete thickness8.
CLINICAL FINDINGS
The temporal region is the predominant location of RAM, although arteries in the nasal region may also be affected3.
In an assessment of the location of RAM in 34 patients, the following percentages were obtained: 50% in the superior temporal region, 44.7% in the inferior temporal region, and 5.2% in the nasal vessels10.
Similar findings were obtained in another study, in which 21 symptomatic RAMs were evaluated, with the following distribution: 52.4% in the superior temporal region, 38% in the inferior temporal region, and 9.6% in the nasal region11.
Macroaneurysms may develop less commonly in the cilioretinal artery12 (Figure 1).
Other findings of this study were as follows: retinal hemorrhage in 81% of the samples, retinal exudates in 70%, vitreous hemorrhage in 30%, macular involvement in 30%, and distal arteriolar narrowing in 26%. Spontaneous arteriolar occlusion occurred in 8% samples and in 16% samples after laser photocoagulation5.
The visual acuity distribution among 60 patients with RAM was as follows: 23% with 20/40 or better, 18% from 20/50 to 20/80, 43% worse than 20/200, and 28% at counting fingers. Half of the patients had a hemorrhagic presentation, and 10% of these had vitreous hemorrhage and 39% presented sub- or preretinal hemorrhage. Macular edema was found in 33% of the cases6. The presence of hemorrhage at multiple levels (retinal, subretinal, and preretinal or vitreous) strongly suggests the presence of RAM, although it may also occur in cases of agerelated macular degeneration (AMD) of the exudative type7 (Figure 2).
DIAGNOSIS AND ADDITIONAL EXAMS
In most cases, arterial macroaneurysms can be diagnosed by clinical examination of the fundus. Fluorescein angiography and optical coherence tomography (OCT) can also assist in the investigation.
Fluorescein angiography
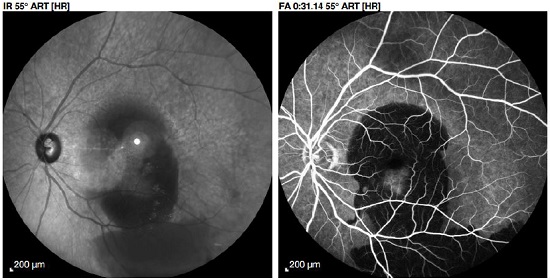
The macroaneurysm is filled with contrast early during the angiogram, presenting itself as a fusiform hyperfluorescence in the shape of a light bulb (Figure 3). In the late phases, its appearance varies from contrast impregnation of the vessel wall to extravasation.
Changes in the retinal capillaries can be observed by fluorescein angiography. Some RAMs present a visible pulsation. The significance of this finding concerning the risk for hemorrhagic complications is uncertain13.
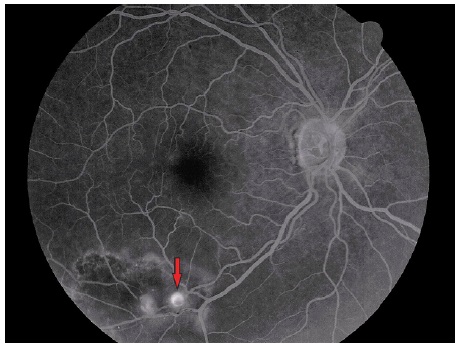
Vitreous hemorrhage from RAM has been documented during retinal fluorescein angiography. Some eyes exhibit yellow plaques (atheroma) near RAM and other retinal arteries (Figure 4).
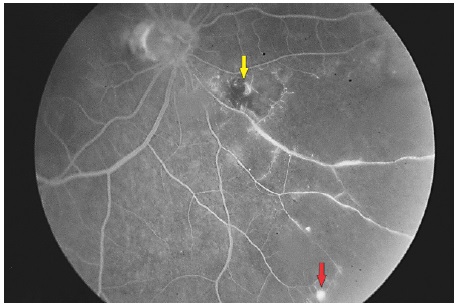
When associated with retinal venous occlusions, retinal ischemia and neovascularization areas may be observed, characterized by progressive leakage5 (Figure 5).
Indocyanine green angiography
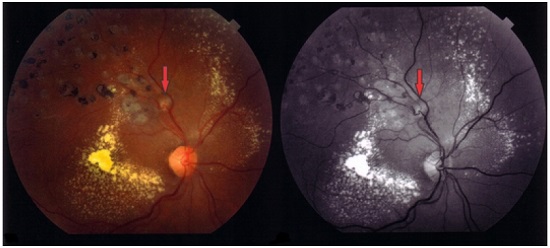
Fluorescein angiography examination is often hindered by the blocking caused by the adjacent hemorrhage that may cover or surround the macroaneurysm (Figure 6). In half of these cases, the diagnosis can be made by fluorescein angiography alone; however, with the use of indocyanine green, which is able to cross the hemorrhagic block, 75%–100% RAMs are diagnosed, showing a pulsatile arterial dilation15-17.
Optical Coherence Tomography (OCT)
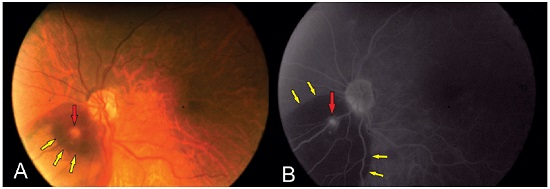
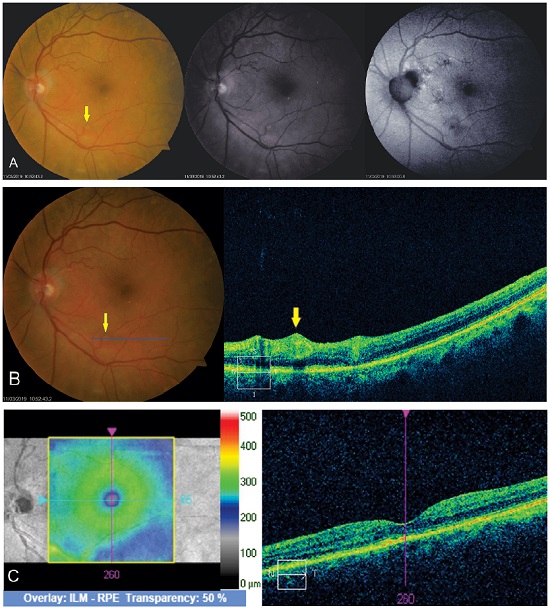
OCT of the lesion shows thickening of the area with increased vascular lumen. The macroaneurysm can be objectively identified and measured (Figure 7).
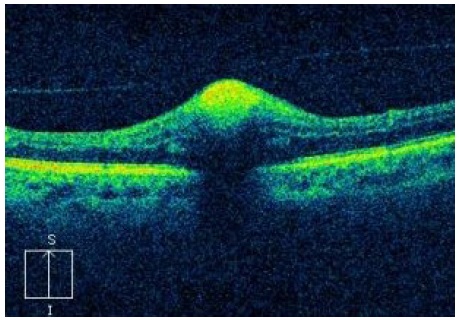
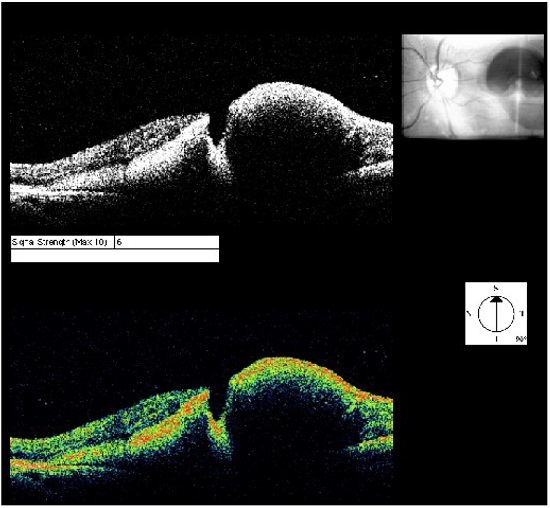
When the macroaneurysm is surrounded by hemorrhage, it is possible to notice the contrast between the raised area and macroaneurysm, similar to a valley between two summits (Figure 8).
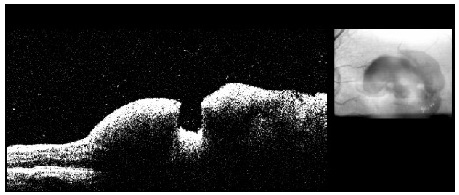
This examination is of great importance to identify complications such as macular edema and serous retinal detachment, which are related to a worse visual prognosis owing to the possibility of irreversible lesion of the retinal outer layers (Figure 9).
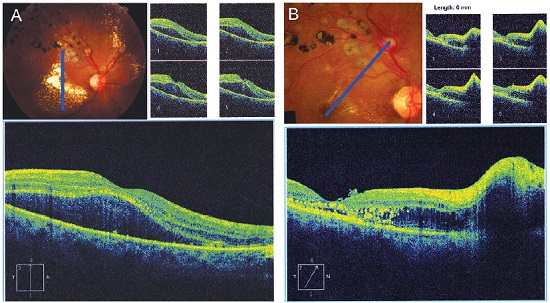
Subretinal hemorrhages are shown with hyperreflectivity on the examination18,19 (Figure 10).
Angio-OCT allows observing the flow inside RAM, which distinguishes thrombosed RAM from patent RAM20.
DIFFERENTIAL DIAGNOSIS
Various clinical presentations of RAM with exudation and hemorrhage at many levels (pre-, sub- or intraretinal) can simulate several conditions, such as other retinal vasculopathies, diabetic retinopathy, vein occlusions, some tumors (including retinal hemangiomas), choroid melanoma, and exudative AMD, which characterizes a “masked syndrome”21,22. Making a differential diagnosis becomes even more difficult because of the presence of vitreous or preretinal hemorrhage obscuring the lesion. Ultrasonography may reveal a lesion with tumor appearance, in which other typical characteristics of linear ultrasound (mode A) of choroid hemangiomas and melanomas are absent. The internal reflectivity is average and heterogeneous in the presence of massive subretinal hemorrhage associated with RAM.
Fluorescein angiography reveals the presence of hemorrhage around an artery with a typical dilated and hyperfluorescent lesion in the shape of a light bulb, contributing to the diagnosis of RAM. The presence of multiple-level hemorrhage in one lesion suggests the presence of RAM, exudative AMD, and/or ischemic retinal vein occlusion.
OTHER ASSOCIATIONS
A syndrome characterized by retinal vasculitis, multiple macroaneurysms, neuroretinitis, and peripheral retinal ischemia (idiopathic retinal vasculitis, aneurysms, and neuro-retinitis) showed a correlation with RAM and other retinal findings. However, no systemic associations that contributed to the diagnosis were found23.
PATHOLOGY
RAM solely comprises an anomaly of the arterial structure. Narrowing of the distal artery can be observed along with occasional occlusion of the arterial branch. Exudation and bleeding to the adjacent retina may also occur, along with protein deposits, lipid material, and hemosiderin in areas adjacent to RAM, retina, and subretinal space. Retinal capillaries are dilated around them. Clinically, these complications may lead to macular edema, serous retinal detachment, lipid deposition in a circinate pattern, and intra-retinal hemorrhages around the aneurysms7,8.
TREATMENT AND PROGNOSIS
Natural history with satisfactory evolution without treatment has been widely studied, making the treatment of RAM a controversial subject24,25. Because many patients are asymptomatic and others show good progression with monitoring alone (Figure 11), the indication for treatment is restricted to complications such as macular exudation and hemorrhages with macular involvement or decreased vision26,27.
Laser photocoagulation was one of the first treatments to be described with satisfactory results. Lasers with greater absorption by hemoglobin are usually preferred, such as those with wavelengths in the yellow spectrum, although therapeutic success has also been reported with other types of laser28-31.
The proposed treatment is occlusion of the macroaneurysm. To this end, the suggestion is to continuously circle the lesion with wide beams (200–500 microns) and moderate intensity, possibly followed by direct application on the surface of the macroaneurysm with mild intensity and a beam of the size of the lesion28.
Although conservative treatment is advocated by authors who have reported excellent prognosis with thrombosis and resorption of exudates, retinal structural damage may occur owing to edema or hemorrhage, leading to permanent low vision. There is no evidence supporting the advantage of using photocoagulation to treat macroaneurysms28.
Although photocoagulation is indicated when exudation threatens the macula, complications such as vascular occlusion may occur, which are more severe the closer they are to the central area28-31.
Anti-vascular endothelial growth factor therapy has been used alone or in association with photocoagulation for patients presenting with macular edema45-47. This treatment is safe and yields satisfactory results by reducing hemorrhage and edema (measured by OCT). It also improves visual acuity with only one application, in most cases32.
Nd:Yag laser can also be used in cases of preretinal hemorrhage to perforate the posterior hyaloid and drain the hemorrhage into the vitreous cavity, clearing the visual axis and providing rapid improvement of the vision35. Although it can be performed in outpatient units, complications such as retinal perforation as well as development of epiretinal membranes and macular holes may occur35-37.
The isolated injection of gas into the vitreous cavity can also be used for downward displacement of the hemorrhage to attempt the preservation of the photoreceptors and RPE in the macular region38. However, this technique should be performed within 24 hours of the appearance of the macroaneurysm, with the patient in the face-down position for 5 days for best results38.
Pars plana vitrectomy has been reported as an option for the treatment of vitreous hemorrhage, both subhyaloidal and subretinal39. The use of adjuvants, such as tissue plasminogen activator, associated with pneumatic displacement of the hemorrhage or not 39 has been reported to facilitate the removal of subretinal blood, which is potentially toxic to the retinal pigment epithelium and photoreceptors and requires surgical removal39-44. In addition, vitrectomy can be diagnostic and therapeutic in cases of persistent vitreous hemorrhage, allowing the application of endophotocoagulation during surgery (Figure 12).
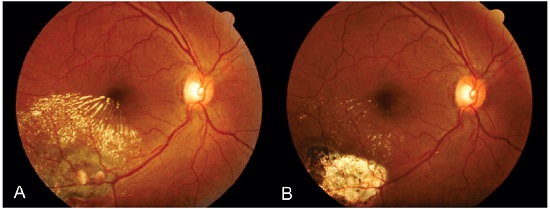
Figure 12. Postvitrectomy fundus photography (A) with endophotocoagulation for the treatment of vitreous hemorrhage and lipid exudation secondary to retinal arterial macroaneurysm. After 7 months of treatment (B), there was a significant reduction in retinal edema and hard exudates, and vision was regained.
In rare cases, rhegmatogenous retinal detachment, subretinal neovascular membranes, and macular holes, associated with subretinal hemorrhage or not, were reported as complications associated with the rupture of RAM45.
REFERENCES
1. Mandava N, Yannuzzi LA. Miscellaneous Retinal Vascular Conditions. In: Brown GC, Flynn HW Jr, Regillo CD. (Eds.). Vitreoretinal Disease: The Essentials. New York: Thieme Medical Publishers, Inc; 1999. p.204-206.
2. Robertson DM. Macroaneurysms of the retinal arteries. Trans Am Acad Ophthalmol Otolaryngol.1973 jan/feb;77(1):55-67.
3. Green WR. Retinal Ischemia: vascular and circulatory conditions and diseases. In: Spencer WH. (Ed.). Ophthalmic Pathology. Philadelphia: WB Saunders; 1985. p.102-12.
4. Bopp S. Retinal Arterial Macroaneurysms. In: Joussen AM, Gardner TW, Kirchhof B, Ryan SJ. (Eds.). Retinal Vascular Disease. New York: Springer- Verlag Berlin Heidelberg; 2007. p.543-560.
5. Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol. 1990 oct;74(10):595-600.
6. Cousins SW, Flynn HW Jr, Clarkson JG. Macroaneurysms associated with retinal branch vein occlusion. Am J Ophthalmol. 1990;109(5):567-70.
7. Rabb MF, Gagliano DA, Teske MP. Retinal arterial macroaneurysms. Surv Ophthalmol. 1988 sep/oct;33(2):73-96.
8. Fichte C, Streeten BW, Friedman AH. A histopathologic study of retinal arterial aneurysms. Am J Ophthalmol. 1978;85(4):509-18.
9. Perry HD, Zimerman LE, Benson WE. Hemorrhage from isolated aneurysm of a retinal artery: report of two cases simulating malignant melanoma. Arch Ophthalmol. 1977 feb;95(2):281-3.
10. Moosavi RA, Fong KC, Chopdar A. Retinal artery macroaneurysms: clinical and fluorescein angiographic features in 34 patients. Eye (Lond). 2006 sep;20(9):1011-20.
11. Tezel TH, Gunalp I, Tezel G. Morphometric analysis of exudative retinal arterial macroaneurysms: a geometrical approach to exudate curves. Ophthalmic Res. 1994;26(6):332-9.
12. Morizot E, Silva D. Macroaneurisma de artéria cílio-retiniana / Cilio retinal macroaneurysm. Rev Bras Oftalmol. 1991 out;50(5):285-288.
13. Bleckmann H. Pulsating macroaneurysm of a retinal arterial branch. Klin Monbl Augenheilkd. 1983 jan;182(1):91-3.
14. Territo JG, Rose SJ, Lindahl KJ. Spontaneous rupture of a macroaneurysm documented in real time during fluorescein angiography. Arch Ophthalmol. 2000 jan;118(1):144-5.
15. Townsend-Pico WA, Meyers SM, Lewis H. Indocyanine green angiography in the diagnosis of retinal arterial macroaneurysms associated with submacular and preretinal hemorrhages: a case series. Am J Ophthalmol. 2000 jan;129(1):33-7.
16. Gomez-Ulla F, Gonzalez F, Torreiro MG, Perez R, Des J. Indocyanine green angiography in isolated primary retinal arterial macroaneurysms. Acta Ophthalmol Scand.1998 dec;76(6):671-4.
17. Schneider U, Wagner AL, Kreissig I. Indocyanine green videoangiography of hemorrhagic retinal arterial macroaneurysms. Ophthalmologi ca.1997;211(2):115-8.
18. Takahashi K. Kishi S. Serous macular detachment associated with retinal arterial macroaneurysm. Jpn J Ophthalmol. 2006 sep/oct;50(5):460-4.
19. Savar A, Vavvas D. Optical coherence tomography appearance of a retinal artery macroaneurysm. Ophthalmic Surg Lasers Imaging. 2009 jul/ aug;40(4):403-4.
20. Manzanaro PG, Santiago-Balsera H, Sastre IV. Angio-OCT en macroaneurisma arterial retiniano. Arch Soc Esp Oftalmol. 2017 march;92(3):e17. Available from: http://dx.doi.org/10.1016/j.oftal.2016.11.001
21. Shields CL, Shields JA. Subretinal hemorrhage from a retinal arterial macroaneurysm simulating a choroidal melanoma. Ophthalmic Surg Lasers. 2001 jan/feb;32(1):86-7.
22. Spalter HF. Retinal macroaneurysms: a new masquerade syndrome. Trans Am Ophthalmol Soc. 1982;80:113-30.
23. Chang TS, Aylward GW, Davis JL, Mieler WF, Oliver GL, Maberley AL, Gass JD. Idiopathic retinal vasculitis, aneurysms, and neuro-retinitis. Retinal Vasculitis Study. Ophthalmology. 1995 jul;102(7):1089-97.
24. Cleary PE, Kohner EM, Hamilton AM, Bird AC. Retinal macroaneurysms. Br J Ophthalmol. 1975;59(7):355-61.
25. McCabe CM, Flynn HW Jr, McLean WC, Brod RD, McDonald HR, Johnson MW, Williams GA, Mieler WF. Nonsurgical management of macular hemorrhage secondary to retinal artery macroaneurysms. Arch Ophthalmol. 2000 jun;118(6):780-5.
26. Alezzandrini AA, Collado P. Macroaneurismas de la retina: importancia clínica y tratamiento / Retinal arteries macroaneurysms: clinical importance and treatment. Arch Oftalmol B Aires. 1986 jul/set;61(3):228-232.
27. Gonçalves JCM, Bonomo PP. Macroaneurismas arteriais retinianos adquiridos / Acquired arterial retinal macroaneurysms. Arq Bras Oftalmol. 1996 abr;59(2):156-160.
28. Joondeph BC, Joondeph HC, Blair NP. Retinal macroaneurysms treated with the yellow dye laser. Retina. 1989;9(3):187-92.
29. Parodi MB, Iacono P, Ravalico G, Bandello F. Subthreshold laser treatment for retinal arterial macroaneurysm. Br J Ophthalmol. 2011 apr;95(4):534-8.
30. Dragon DM, Robin AL, Pollack IP, Quigley HA, Green WR, Murray TG, Hotchkiss ML, D’Anna S. Neodymium:YAG laser iridotomy in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1985 jun;26(6):789-96.
31. Psinakis A1, Kokolakis S, Theodossiadis PG, Koutsandrea C. [Pulsatile arterial macroaneurysm: management with argon laser photocoagulation]. J Fr Ophtalmol. 1989;12(10):673-6.
32. Chen YY, Lin LY, Chang PY, Chen FT, Mai ELC, Wang JK. Laser and Anti-Vascular Endothelial Growth Factor Agent Treatments for Retinal Arterial Macroaneurysm. Asia Pac J Ophthalmol (Phila). 2017 sep/oct;6(5):444-49.
33. Bormann C, Heichel J, Hammer U, Habermann A, Hammer T. Intravitreal Anti-Vascular Endothelial Growth Factor for Macular Edema due to Complex Retinal Arterial Macroaneurysms. Case Rep Ophthalmol. 2017 jan/apr;8(1):137-43.
34. Chatziralli I, Maniatea A, Koubouni K, Parikakis E, Mitropoulos P. Intravitreal ranibizumab for retinal arterial macroaneurysm: long-term results of a prospective study. Eur J Ophthalmol. 2017 mar;27(2):215-19.
35. Iijima H, Satoh S, Tsukahara S. Nd:YAG laser photodisruption for preretinal hemorrhage due to retinal macroaneurysm. Retina. 1998;18(5):430-4.
36. Raymond LA. Neodymium:YAG laser treatment for hemorrhages under the internal limiting membrane and posterior hyaloid face in the macula. Ophthalmology. 1995 mar;102(3):406-11.
37. Tassignon MJ, Stempels N, Van Mulders L. Retrohyaloid premacular hemorrhage treated by Q-switched Nd-YAG laser. A case report. Graefes Arch Clin Exp Ophthalmol. 1989;227(5):440-2.
38. Abdelkader E, Yip KP, Cornish KS. Pneumatic displacement of submacular haemorrhage. Saudi J Ophthalmol. 2016 oct/dec;30(4):221–226.
39. Brent BD, Gonce M,Diamond JG. Pars plana vitrectomy for complications of retinal arterial macroaneurysms - a case series. Ophthalmic Surg. 1993 aug;24(8):534-6.
40. Humayun M, Lewis H, Flynn HW Jr, Sternberg P Jr, Blumenkranz MS. Management of submacular hemorrhage associated with retinal arterial macroaneurysms. Am J Ophthalmol. 1998 sep;126(3):358-61.
41. Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007 mar;27(3):321-8.
42. Hassan AS, Johnson MW, Schneiderman TE, Regillo CD, Tornambe PE, Poliner LS, Blodi BA, Elner SG. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999 oct;106(10):1900-6; discussion 1906-7.
43. Peyman GA, Nelson NC Jr, Alturki W, Blinder KJ, Paris CL, Desai UR, Harper CA 3rd. Tissue plasminogen activating factor assisted removal of subretinal hemorrhage. Ophthalmic Surg. 1991 oct;22(10):575-82.
44. Hesse L, Kroll P. Pneumatic displacement of submacular hemorrhage. Ophthalmology. 2000 dec;107(12):2119-20.
45. Vianna RN, Kassuga A, Onofre G, Ecard V, Burnier MN Jr. Subretinal neovascularization following ruptured retinal arterial macroaneurysm: case report. Arq Bras Oftalmol. 2007 jul/ago;70(4):698-700.
46. Tashimo A, Mitamura Y, Ohtsuka K, Okushiba U, Imaizumi H, Takeda M. Macular hole formation following ruptured retinal arterial macroaneurysm. Am J Ophthalmol. 2003 apr;135(4):487-92.
47. Tashimo A, Mitamura Y, Sekine N, Takeda M, Ohtsuka K. Rhegmatogenous retinal detachment after rupture of retinal arterial macroaneurysm. Am J Ophthalmol. 2003 sep;136(3):549-51.




Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
October 26, 2018.
Accepted on:
May 22, 2019.