Roberta de Julio Matheus1; Priscilla Fernandes Nogueira2; Marcello Novoa Colombo Barboza3; Julia Alves Utyama4; Marta Fabiane Gouvêa Barioni5
DOI: 10.17545/eoftalmo/2018.0037
ABSTRACT
Elevation of intraocular pressure in the form of crises, which are commonly observed in ophthalmological practice, may be unilateral or bilateral and involves a series of potential differential diagnoses. Some patients progress to glaucoma; in these cases, correct diagnosis and management of the condition is essential to prevent damage. This study reports two separate cases of Posner-Schlossman syndrome (PSS) pigment dispersion syndrome (PDS) and compares the findings, focusing on the differential diagnosis. PSS and PDS have similar characteristics, and both present a risk for glaucoma development, a fact that reinforces the need for effective patient follow-up.
Keywords: Glaucoma; Open-Angle; Ocular Hypertension; Uveitis; Anterior; Intraocular Pressure.
RESUMO
O aumento da pressão intraocular em forma de crises é muito comum na prática oftalmológica, podendo ser unilateral ou bilateral, envolvendo uma série de diagnósticos diferenciais possíveis. Em alguns casos ocorre progressão para glaucoma, sendo assim, o correto diagnóstico e manejo desses pacientes necessário a fim de evitar os seus danos. O objetivo desse estudo foi relatar um caso de Síndrome de Posner-Schlossman (SPS) e outro de Síndrome de Dispersão Pigmentar (SDP) comparando seus achados e ênfase no diagnóstico diferencial. A SPS e SDP são duas entidades com características semelhantes e ambas apresentam risco para desenvolvimento de glaucoma, reforçando a necessidade de seguimento efetivo dos pacientes.
Palavras-chave: Glaucoma de Ângulo Aberto; Hipertensão Ocular; Uveíte Anterior; Pressão Intraocular.
RESUMEN
El aumento de la presión intraocular en forma de crisis es comúnmente encontrado en la práctica oftalmológica, pudiendo ser unilateral o bilateral, involucrando una serie de diagnósticos diferenciales posibles. En algunos casos, ocurre progresión para glaucoma, por ello, es necesario el correcto diagnóstico y manejo de dichos pacientes, con la finalidad de evitar daños. El objetivo de este estudio fue relatar un caso de Síndrome de Posner-Schlossman (SPS) y otro de Síndrome de Dispersión Pigmentaria (SDP) comparando sus hallazgos y énfasis en el diagnóstico diferencial. Los SPS y SDP son dos entidades con características semejantes y ambos presentan riesgo para desarrollo de glaucoma, reforzando la necesidad de seguimiento efectivo de los pacientes.
Palabras-clave: Glaucoma de Ángulo Abierto; Hipertensión Ocular; Uveítis Anterior; Presión Intraocular.
INTRODUCTION
Acute unilateral elevation of intraocular pressure (IOP) occurs commonly and is frequently reported in ophthalmological emergency settings1. It may occur as an isolated episode or as crises. Because it involves several potential differential diagnoses, including Posner-Schlossman syndrome (PSS) and pigment dispersion syndrome (PDS), it is essential to know how to differentiate between them.
PSS, also known as glaucomatocyclitic crisis, is a recurrent inflammatory ocular condition2 that is characterized by unilateral crises of IOP elevation that lasts from a few hours to several weeks, with IOP attaining elevated values (40 mmHg). It affects young male adults and its etiology remains unknown3. Clinical manifestations may include mild uveitis and some visual acuity symptoms, such as halos or blurry vision, which make its diagnosis more challenging2.
PDS is characterized by pigment dispersion caused by friction between the iris pigment epithelium and the zonules and anterior lens capsule, which leads to pigment deposition on the trabecular meshwork and results in progressive trabecular dysfunction and IOP elevation4. Physical exercise and mydriasis are precipitating factors of this process. The condition primarily affects white men with myopia4. Fifteen percent of patients with PDS develop pigmentary glaucoma up to 15 years after diagnosis. The most common presentation is bilateral and asymmetric PDS but unilateral PDS may also manifest1, with elevated IOP and pigments detected on biomicroscopy1.
The objective of this study was to report separate cases of PSS and PDS, focusing on the importance of a differential diagnosis and understanding how to differentiate the conditions for adequate patient management.
CASE REPORTS
Case1: A 36-year-old white emmetropic man complaining of irritation and pain and seeing colored halos around lights in the left eye (LE) for 3 years. History: lumbago. On examination: Uncorrected visual acuity (VA) of 20/20 in both eyes (BE); Biomicroscopy (Bio): normal right eye (RE) and fine keratic precipitates, traces of cells, and initial cataracts in the LE; IOP of 16 and 40 mmHg (10:30 h) measured by Goldmann applanation tonometry; Ophthalmoscopy: pale-pink, well-delimited medial nerve, 360° rim and physiological excavation in BE (Figure 1). Prescription: prednisolone acetate 1% combined with Timolol maleate 0.5% and Brinzolamide and tests were requested. After 3 days, IOP: 12 mmHg in BE (10:30 h) measured by Goldmann applanation tonometry, and negative tests for infectious and rheumatological diseases.
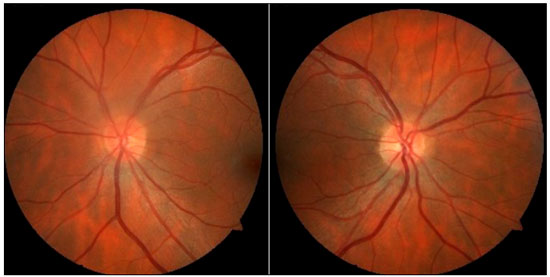
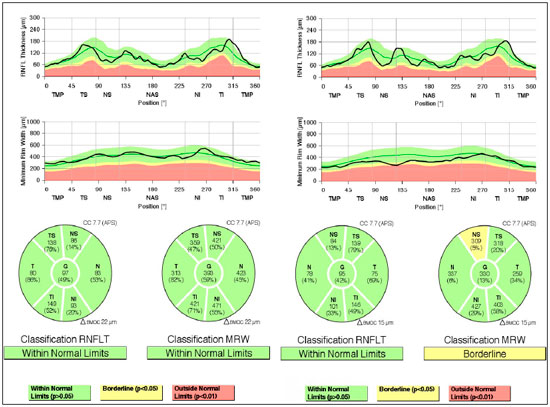
Evaluation of glaucoma: Gonioscopy: revealed posterior trabeculation in all four quadrants with open angle of 360° in BE; OCT: RE: thickness of the nerve fiber layer within normal limits in the papilla and in the fiber region of the macula of retina; LE: thickness of the nerve fiber layer within normal limits in the papilla, thickness of the macula of retina within normal limits in the superior-temporal and inferior-nasal regions, and borderline in the superior-nasal region (Figure 2). PSS was confirmed.
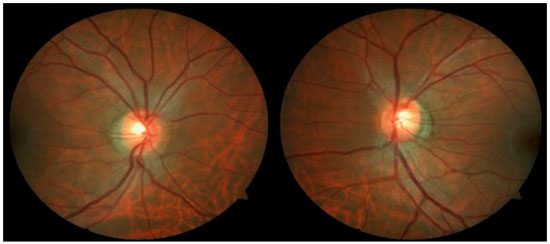
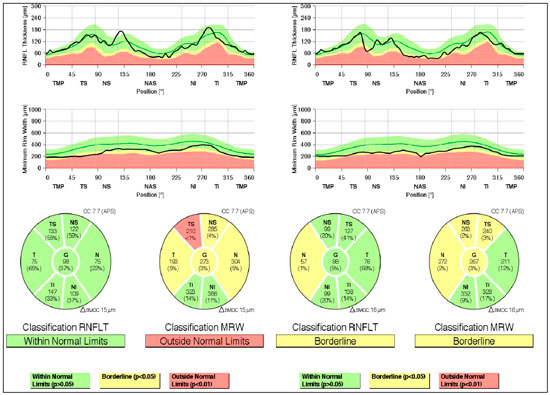
Case 2: A 29-year-old white man with myopia complaining of hyperemia in the RE for 8 months. On examination: best-corrected VA: 20/20 in BE; IOP:16 mmHg BE (09:30 h) measured by Goldmann applanation tonometry; Bio: RE: fine keratic precipitates, anterior chamber reaction +2, posterior synechiae; LE: pigmented fine keratic precipitates; ophthalmoscopy: papilla discretely tilted, medial, pale-pink, and well delimited 360°, excavation 0.3 × 0.3 and peripapillary atrophy BE (Figure 3). Prescription: prednisolone acetate 1% and Tropicamide, and exams were requested. After 2 days, IOP: 26 and 20 mmHg (09:30 h) as measured by Goldmann applanation tonometry, Timolol maleate 0.5% was added, negative tests for infectious and rheumatological diseases, glaucoma was assessed due to presence of pigments in the corneal endothelium, with a diagnosis of SDP. Gonioscopy: revealed posterior trabeculation with dark brown pigment in all four quadrants in BE. OCT: RE: thickness of the nerve fiber layer within normal limits in the papilla and in the fiber area of the macula of retina; borderline thickness in the temporal-nasal and superior-nasal regions; thickness outside normal limits in the temporal-superior region and normal in the remaining regions; LE: thickness of nerve fiber layers within normal limits in all regions of the papilla, except in the nasal region where it is borderline and in the fiber region of the retina; borderline thickness of the macula in the superior-nasal and superior-temporal regions (Figure 4); Visual field: nonspecific scotomas in BE (Figure 5)
DISCUSSION
When encountering a young adult male patient with unilaterally elevated IOP and without significant ocular findings, potential differential diagnoses should be considered, including PSS and PDS. Both syndromes affect this population and their clinical presentations are similar. PDS may also occur in myopic individuals and present bilateral involvement4,5. On ocular physical examination, PSS and PDS exhibit characteristics such as small keratic precipitates, which may be pigmented in PDS5,7. In addition, Krukenberg’s spindle and iris transillumination may occasionally manifest in PDS7.
The diagnosis and follow-up of these patients are extremely important owing to the rate of progression to glaucoma and subsequent findings, such as loss of visual field sensitivity, loss of nerve fibers, and optic nerve changes. PSS is associated with the development of primary open-angle glaucoma9,11, consequently resulting in loss of the retinal nerve fiber layer, which is directly associated with the duration of the ocular hypertension crises8. The degeneration mechanism of the nerve fiber layer in PSS is called secondary degeneration, in which neuropathy continues to develop even after adequate IOP reduction8. Moreover, during episodes of ocular hypertension, the head of the optic nerve undergoes morphological changes and hemodynamic variations6. Patients who experience recurrent crises over 10 years or more have a 2.8-fold higher risk of developing glaucoma than patients who have the disease for less than 10 years8. Thus, correct diagnosis and management of these crises is important to prevent progression and the development of glaucoma and subsequent damage8. Ocular antiglaucomatous agents and topical steroids can be used during a crisis; however, these do not prevent future attacks5. Surgery may be indicated for more advanced cases with alterations in the optic nerve and visual field6.
In PDS, the rate of progression to pigmentary glaucoma is approximately 35%–50%7,10. PDS occurs as a result of chronic pigment shedding that is deposited on the trabecular meshwork, resulting in IOP elevation4, and the degree of pigmentation is associated with disease severity. Risk factors for progression to pigmentary glaucoma include initial IOP >21 mmHg, a family history of glaucoma, male gender, myopia, and presence of Krukenberg’s spindle12. This syndrome is managed by IOP control with hypotensive agents alone or combined; miotics have the advantage of decreasing IOP and eliminating the posterior concavity of the iris, thereby reducing iridozonular contact4; however, their great disadvantage is that they exacerbate myopia and are therefore difficult to use4. Laser trabeculoplasty or trabeculectomy can be used in cases of clinical treatment failure 4.
PDS and PSS possess specific characteristics, and their diagnosis may be challenging because of negative laboratory test results and because these syndromes are only associated with elevated IOP. The manifestations seen in these two cases facilitated establishment of a differential diagnosis between the two syndromes associated with the risk of developing glaucoma and subsequent damage. In both cases, it was possible to observe an abnormal pattern in the nerve fiber layer, which indicates glaucomatous damage and the need for effective follow-up.
REFERENCES
1. Lopes AS, Silva D, Mota M, Pedrosa CA, Vendrell MC, Lisboa M, Vaz FT. Síndrome de dispersão pigmentar ou síndrome de Posner-Schlossman? Um desafio diagnóstico. Apresentação no 58° Congresso Português de Oftalmologia. Lisboa: Portugal; 2015.
2. Green RJ. Posner-Schlossman syndrome (glaucomatocyclitic crisis). Clin Exp Optom. 2007 Jan; 90(1):53-6.
3. Shazly TA, Aljajeh M, Latina MA. Posner–Schlossman glaucomato cyclitic crisis. Semin Ophthalmol. 2011 Jul-Sep; 26(4-5):282-4.
4. Silva AM, Fialho R, Braz F, Dias JA, Fernandes J, Coelho A, Coutinho-Santos L. Síndrome de dispersão pigmentar: abordagens diagnósticas e terapêuticas. Oftalmologia. 2012 Abr-Jun; 36:141-5.
5. Takusagawa HL, Liu Y, Wiggs JL. Infectious theories of Posner-Schlossman syndrome. Int Ophthalmol Clin. 2011 Fall; 51(4):105-15.
6. Darchuk V, Sampaolesi J, Mato L, Nicoli C, Sampaolesi R. Optic nerve head behavior in Posner-Schlossman syndrome. Int Ophthalmol. 2001; 23(4- 6):373-9.
7. Sivaraman KR, Patel CG, Vajaranant TS, Aref AA. Secondary pigmentary glaucoma in patients with underlying primary pigment dispersion syndrome. Clin Ophthalmol. 2013; 7:561-6.
8. Tsai JC. Detection of the progression of retinal nerve fiber layer loss by optical coherence tomography in a patient with glaucomatocyclitic crisis. Taiwan J Ophthalmol. 2015 Apr-Jun; 5(2):90-3.
9. Kim T, Kim J, Kee C. Optic disc atrophy in patient with Posner-Schlossman syndrome. Korean J Ophthalmol. 2012 Dec; 26(6):473-7.
10. Okafor K, Vinod K, Gedde J. Update on pigment dispersion syndrome and pigmentary glaucoma. Curr Opin Ophthalmol. 2017 Mar; 28(2):154-60.
11. Silveira CG, Oliveira PR, Silveira RM. Síndrome de Posner-Schlossman. Retato de três casos. Rev Bras Oftalmol. 2010; 69(4):269-75.
12. Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma – a major review. Clin Exp Ophthalmol. 2008 Dec; 36(9):868-82.




Funding: No specific financial support was available for this study
CEP Approval: Not applicable
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Received on:
October 9, 2018.
Accepted on:
October 31, 2018.