Laurentino Biccas Neto1; André Correa Maia de Carvalho2; Guilherme Sturzeneker Cerqueira Lima3
DOI: 10.17545/e-oftalmo.cbo/2017.89
ABSTRACT
The Brazilian Association of Cataract and Refractive Surgery (ABCCR/BRASCS) chose to present an update on this topic because they currently consider optical coherence tomography (OCT) as an essential resource for the preoperative and postoperative evaluation of patients undergoing elective cataract surgery. In this review, the authors emphasize the important role of OCT in cases in which the expected improvement in visual acuity was not achieved or in the follow-up of patients with age-related macular degeneration and diabetes mellitus because of the possibility of worsening of the macular condition.
Keywords: Cataract extraction; Tomography; Optical coherence; Fovea centralis; Visual acuity.
RESUMO
A Sociedade Brasileira de Catarata e Cirurgia Refrativa (ABCCR/ BRASCS) escolheu este tema para atualização entendendo que a tomografia de coerência óptica (OCT) é hoje um recurso fundamental na avaliação pré e pós-operatória de rotina de todo paciente que será submetido à cirurgia eletiva de catarata. Os autores enfatizam nesta revisão o papel importante que a OCT tem para os casos que não obtiveram a melhora esperada da acuidade visual ou para o acompanhamento dos pacientes com DMRI e DM, pela possibilidade de agravamento da afecção macular.
Palavras-chave: Extração de catarata; Tomografia de coerência óptica; Fóvea central; Acuidade visual.
1. INTRODUCTION
Phakectomy is increasingly seen as a procedure that is safe, functionally successful, and with refractive predictability. Theuse of premium intraocular lenses—aspheric, toric, multifocal—make excellent results and refractive predictability a necessity after phakectomy. Different tests can be performed to measure the final potential of visual acuity after the procedure and other changes that may affect the end result, depending on the situation. These include tests such as the potential acuity meter test and binocular indirect ophthalmoscopy. Spectral domain optical coherence tomography (SD-OCT) is a valuable tool available to surgeons to detect preoperative conditions that may have a negative effect on the desired result and to identify postoperative situations that require intervention. OCT can sometimes indicate the etiology of a cystoid edema postoperatively, when good visualization of the fundus of the eye was not possible before the surgery.
2. PREOPERATIVE SITUATIONS THAT MAY AFFECT THE FINAL VISUAL RESULT
2.1. Changes in the vitreomacular interface
Changes that occur as a result of vitreomacular interface aging may cause problems such as vitreomacular traction syndrome, epiretinal membranes, and full-thickness and lamellar macular holes. If these situations are not identified and treated before cataract treatment, the end result may be compromised. This aspect is particularly important when implanting multifocal intraocular lenses (IOL), which usually decrease the sensitivity to contrast and cause dysphotopsia phenomena that may be even more symptomatic in the presence of macular problems. In addition, the anteriorization of the vitreous body after the removal of the lens may worsen the abovementioned conditions, especially vitreomacular traction.
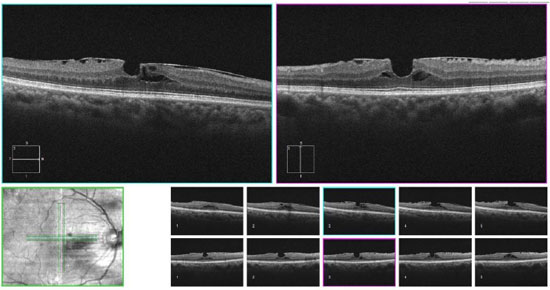
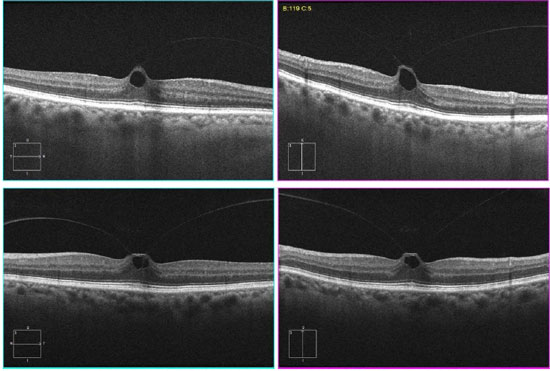
2.2. Synchysis scintillans
This degenerative pathology is historically known as cholesterolosis bulbi,1 and consists in the accumulation of cholesterol crystals in the vitreous humor thatoccurs under specific conditions after breakdown of the blood-ocular barrier, such as ocular trauma, chronic ocular inflammation and long-standing retinal detachment. In this condition, SD-OCT provides more detailed anatomical information on the macula than clinical examination.
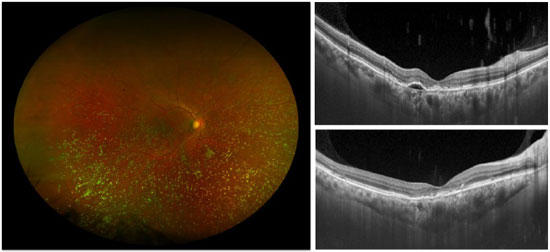
2.3. Age-Related Macular Degeneration (AMD)
AMD and cataract are common causes of visual loss in the aging population. Recent advances in the treatment of exudative AMD have been successful in stabilizing and even improving vision in a large number of cases.2,3,4,5 Therefore, it is not uncommon to see patients with AMD who develop visually significant cataract. However, there is a concern regarding the risk of exacerbating the neovascularization of the choroid or accelerating the progression of geographic atrophy.
Chew et al.6 conducted an analysis of eyes with dry AMD included in the Age-Related Eye Disease Study that underwent phakectomy. The authors defined disease progression by conversion to the exudative form, onset of geographic atrophy or geographic atrophy with central involvement. Results did not demonstrate a clear effect of cataract surgery on disease progression.
With regard to exudative AMD, noncomparative retrospective studies showed visual acuity improvement after cataract surgery, with a transient increase in the mean macular thickness on OCT –associated or not with the presence of cysts –in the first 3 months7 and subsequent progressive return to the initial levels.8 Therefore, the characteristics observed on OCT possibly refrain ophthalmologists from extending the intervals between injections after the surgery. There has not yet been a methodological monitoring using OCT beyond this period and it is still uncertain whether these anatomical changes represent a transient contribution of pseudophakic cystoid macular edema or worsening of exudative AMD. In any case, treatments with anti-VEGF are beneficial in both diseases.9,10,11
Postoperative fluorescein angiography was not performed routinely to distinguish between postoperative cystoid macular edema and choroidal neovascularization secondary to exudative AMD. If these changes represent cystoid macular edema, routine use of topical corticosteroids, subconjunctival corticosteroids, or topical non-steroidal anti-inflammatory drugs (NSAIDs) could probably decrease or eliminate these findings.12,13 Persistent macular edema could have more profound implications than the loss of visual acuity, such as decreased sensitivity to contrast or decreased color perception. The preservation of these capacities is of the utmost importance in cases of advanced exudative AMD withlimited visual potential.
Although the common intraoperative use of intravitreal bevacizumab has been shown to have good visual and tomographic results14,15 these studies did not include a control group, which made it difficult to determine the effectiveness of an intraoperative dose against the need for more intensive therapy in the perioperative period. In addition, a better understanding of the changes observed on OCT may help clarify whether intraoperative therapy with anti-VEGF is superior to the perioperative use of corticosteroids and NSAIDs in eyes with exudative AMD.
The cited studies report that cataract surgery can be performed safely in the context of exudative AMD. However, ourresults reflect a cautious standard of practice, in which only those eyes with stable disease are referred for phakectomy. The clinical applications of these findings should occur in a similar context, considering that the effect of cataract surgery on eyes with exudative AMD has not yet been studied in randomized clinical trials and that such studies would probably be unethical.
2.4. Glaucoma
Opacity of the ocular media by cataracts may hinder the observation of the glaucomatous damage, especially in saucerized optic nerves and in patients with focal lesions. SD-OCT allows a precise and unequivocal diagnosis of these conditions, which may indicate a bad candidate for multifocal lenses or, in cases of advanced glaucoma damage, the need to change the surgical strategy –combining it with antiglaucomatous surgery, using lower pressure parameters in phacoemulsification, or reinforcing preoperative pressure control.
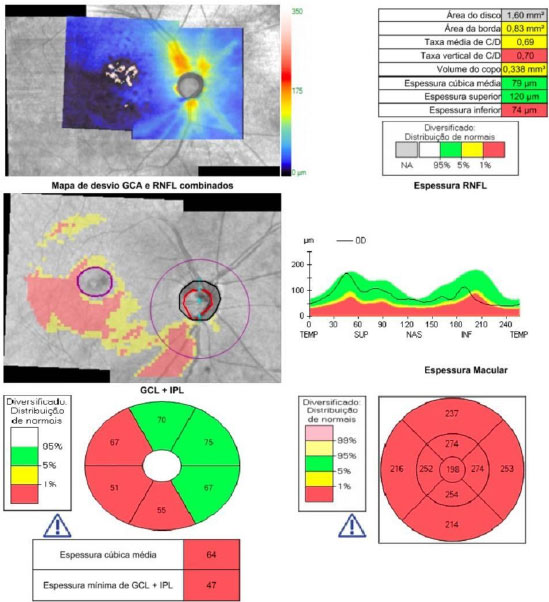
3. POSTOPERATIVE CONDITIONS REQUIRING ATTENTION
3.1. Cystoid edema after phacoemulsification
Cystoid macular edema (CME) after phacoemulsification, also known as Irvine–Gasssyndrome, is a common cause of unexpected visual loss after cataract surgery. The exact pathogenesis of CME is uncertain; however, vascular permeability increased by inflammatory mediators may play a central role.16 According to the current literature, no method has been validated or universally accepted for the diagnosis of pseudophakic CME.17,18 Although fluorescein angiography remains the gold standard,19CME diagnosis can be made clinically by fundus biomicroscopy with a slit lamp and, more recently, by OCT.
Several preoperative and postoperative factors may increase the incidence of CME after phakectomy: trauma of the iris or rupture of the posterior capsule, vitreous loss, iris fixation of the intraocular lens, diabetic retinopathy, retinal vein occlusions, epiretinal membrane, or uveitis.19,20,21,22,23
The Irvine–Gasssyndrome is not frequently found after phacoemulsification performed with small incisions; its incidence ranges from 0.1% to 2.4% when diagnosed clinically, and from 4% to 11% when assessed by OCT.24
However, it is not uncommon to find CME after uncomplicated phacoemulsification in previously healthy eyes.25,26
Clinically, the subsequent visual loss is usually self-limited. However, this condition remains a therapeutic challenge, mainly because the disease may persist for several months with irreversible structural damage, and chronic CME may affect the integrity of the junction of internal and external segments of photoreceptors.
3.2. Diabetic macular edema
Whereas macular edema in Irvine–Gass syndrome is caused by the release of proinflammatory cytokines, in diabetes it is the result of oxidative stress induced by hyperglycemia, deposition of glycated products, poor blood flow, hypoxia, loss of pericytes, loss of endothelial cells, and inflammation.27,28 Most often, the diagnosis is easily determined by anamnesis and clinical findings but this differentiation occasionally becomes difficult, as in the case of diabetic patients with macular edema after cataract surgery.
The morphological appearance of macular edema may depend on the underlying disease. A recent study using SD-OCT29 found more typical characteristics that make the differential diagnosis using imaging exams easier. Increased central macular thickness/retinal volume ratio, absence of epiretinal membrane, and presence of intraretinal cysts predominantly in the internal nuclear layer favor theIrvine–Gass syndrome hypothesis, whereas increase in the external nuclear layer thickness/internal nuclear layer thickness ratio, absence of subretinal fluid, presence of hard exudates or microaneurysms, and cysts concentrated in the ganglion cell layer and/or in the nerve fiber layer reinforce the hypothesis of diabetic edema.
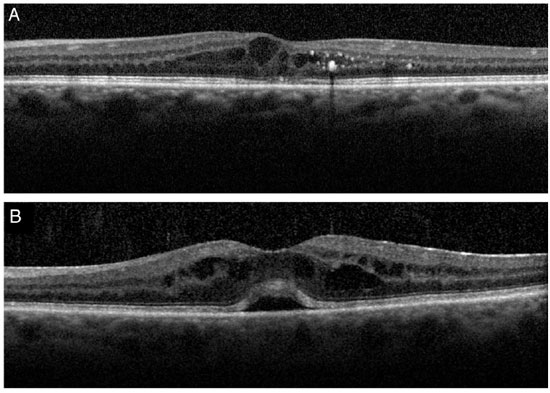
In 2009, Hayashi et al30 performed (Stratus) OCT in diabetic patients who underwent phakectomy with intraocular lens implantation. The final sample was composed of 34 eyes without diabetic retinopathy and 34 eyes with the condition. The exam was performed one day before the surgery and three, six, and 12 months later. Foveal thickness increased in both groups; however, this increasewas more significant in the eyes with retinopathy.
Another study conducted in 2010 using time domain OCT31 compared the foveal thickness of 18 eyes with diabetic retinopathy with that of 36 healthy eyes, both preoperatively and postoperatively (at 1, 7, 30, and 60 days), always using the contralateral eye as a control. Patients with any other ocular pathology were excluded, as well as cases of severe nonproliferative diabetic retinopathy and proliferative forms. An increase in foveal thickness was observed in both groups, which suggests that diabetes did not have a statisticallysignificant effect on macular thickness after uncomplicated cataract surgery.
Therefore, controlled and well-designed clinical studies on this topic are still necessary, especially in the current era of OCT.
4. CONCLUSION
OCT is an essential resource in the routine evaluation of patients who undergo elective cataract surgery. The fact that it is a technically simple, quick, and noninvasive exam further justify its wider use. Moreover, the more popular this method becomes, the lower the cost of its use will be.
In addition to the higher accuracy of OCT during the preoperative assessment, it allows the detailed monitoring of the maculaafter surgery; this is very important in cases in which the expected improvement in visual acuity was not achieved or as a routine exam in patients with AMD and DM because of the possibility of macular deterioration.
REFERENCES
1. Wand M, Smith TR, Cogan DG. Cholesterolosis bulbi: the ocular abnormality known as synchysis scintillans. Am J Ophthalmol 1975;80(2):177-183. Abstract disponível em: https://www.ncbi.nlm.nih.gov/pubmed/1155557
2. Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143(4):566–583. https://doi.org/10.1016/j.ajo.2007.01.028
3. Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, Schneider S, Acharya NR. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 2007;144(6):850–857. https://doi.org/10.1016/j.ajo.2007.08.012
4. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355(14):1419–1431. https://doi.org/10.1056/NEJMoa054481
5. Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008;145(2):239–248. https://doi.org/10.1016/j.ajo.2007.10.004
6. Chew EY, Sperduto RD, Milton RC, Clemons TE, Gensler GR, Bressler SB, Klein R, Klein BE, Ferris FL 3rd. Risk of advanced age-related macular degeneration after cataract surgery in the Age-Related Eye Disease Study: AREDS report 25. Ophthalmology 2009;116(2):297–303. https://doi.org/10.1016/j.ophtha.2008.09.019
7. Muzyka-Wozniak M. Phacoemulsification in eyes with neovascularAMD treated with anti-VEGF injections. Eur J Ophthalmol 2011;21(6):766–770. https://doi.org/10.5301/EJO.2011.6389
8. Grixti A, Papavasileiou E, Cortis D, Kumar BV, Prasad S. Phacoemulsification surgery in eyes with neovascular age-related macular degeneration. ISRN Ophthalmol 2014;2014: 417603. https://doi.org/10.1155/2014/417603
9. Arevalo JF, Garcia-Amaris RA, Roca JA, Sanchez JG, Wu L, Berrocal MH, Maia M; Pan-American Collaborative Retina Study Group. Primary intra-vitreal bevacizumab for the management of pseudophakic cystoid macular edema: pilot study of the Pan-American Collaborative Retina Study Group. J Cataract Refract Surg 2007;33(12):2098–2105. https://doi.org/10.1016/j.jcrs.2007.07.046
10. Arevalo JF, Maia M, Garcia-Amaris RA, Roca JA, Sanchez JG, Berrocal MH, Wu L; Pan-American Collaborative Retina Study Group. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology 2009;116(8):1481–1487. 1487.e1. http://dx.doi.org/10.1016/j.ophtha.2009.04.006
11. Ghasemi Falavarjani K, Parvaresh M-M, Modarres M, Hashemi M, Samiy N. Intravitreal bevacizumab for pseudophakic cystoid macular edema; a systematic review. J Ophthalmic Vis Res 2012;7(3):235–239. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3520593/
12. Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatmentof acute pseudophakic cystoid macular edema. Ophthalmology 2000;107(11):2034–2038. discussion 2039. http://dx.doi.org/10.1016/S0161-6420(00)00365-1
13. Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M; AcularLS for Cystoid Macular Edema (ACME) Study Group. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol 2008;146(4):554–560. https://doi.org/10.1016/j.ajo.2008.04.036
14. Furino C, Ferrara A, Cardascia N, Besozzi G, Alessio G, Sborgia L, Boscia F. Combined cataract extraction and intravitreal bevacizumab in eyes with choroidal neovascularization resulting from age-related macular degeneration. J Cataract Refract Surg 2009;35(9):1518–1522. https://doi.org/10.1016/j.jcrs.2009.04.032
15. Jonas JB, Spandau UHM, Schlichtenbrede F, Libondi T, Vossmerbaeumer U, von Baltz S. Intravitreal bevacizumab combined with cataract surgery for treatment of exudative macular degeneration. J Ocul Pharmacol Ther 2007;23(6):599–600. https://doi.org/10.1089/jop.2007.0050
16. Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin 2010;50:139–153. https://doi.org/10.1097/IIO.0b013e3181c551da
17. Kim SJ, Bressler NM. Optical coherence tomography and cataract surgery. Curr Opin Ophthalmol 2009;20:46–51. https://doi.org/10.1097/ICU.0b013e3283199162
18. Kim SJ, Belair ML, Bressler NM, Dunn JP, Thorne JE, Kedhar SR, Jabs DA. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 2008;28:870–876. https://doi.org/10.1097/IAE.0b013e318169d04e
19. Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol 2012;23:26–32. https://doi.org/10.1097/ICU.0b013e32834cd5f8
20. Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica 2012;227:61–67. https://doi.org/10.1159/000331277
21. Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 2009;23:389–39. https://doi.org/10.1038/sj.eye.6703022
22. Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 2007;33:1550–1558. https://doi.org/10.1016/j.jcrs.2007.05.013
23. Bélair ML, Kim SJ, Thorne JE, Dunn JP, Kedhar SR, Brown DM, Jabs DA. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol 2009;148:128–135. https://doi.org/10.1016/j.ajo.2009.02.029
24. Perente I, Utine CA, Ozturker C, Cakir M, Kaya V, Eren H, Kapran Z, Yilmaz OF. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res 2007;32:241–247. https://doi.org/10.1080/02713680601160610
25. Kusbeci T, Eryigit L, Yavas G, InanUU. Evaluation of cystoid macular edema using optical coherence tomography and fundus fluorescein angiography after uncomplicated phacoemulsification surgery. Curr Eye Res 2012;37:327–333. https://doi.org/10.3109/02713683.2011.635402
26. Vukicevic M, Gin T, Al-Qureshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Experiment Ophthalmol 2012;40:282–287. https://doi.org/10.1111/j.1442-9071.2011.02638.x
27. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. https://doi.org/10.1016/j.survophthal.2008.10.001
28. Bandello F, Battaglia Parodi M, Lanzetta P, Loewenstein A, Massin P, Menchini F, Veritti D. Diabetic macular edema. Dev Ophthalmol. 2010;47:73–110. https://doi.org/10.1159/000320075
29. Munk MR, Jampol LM, Simader C, Huf W, Mittermüller TJ, Jaffe GJ, Schmidt-Erfurth U. Differentiation of diabetic macular edema from pseudophakic cystoid macular edema by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci., 2015;56(11):6724-33. https://doi.org/10.1167/iovs.15-17042
30. Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular edema after phacoemulsication surgery. Eye (Lond). 2009;23(2):389-96. https://doi.org/10.1038/sj.eye.6703022
31. BiróZ, Balla Z. OCT measurements on the foveal and perifoveal retinal thickness on diabetic patients after phacoemulsi cation andIOL implantation. Eye (Lond). 2010;24(4):639-47. https://doi.org/10.1038/eye.2009.164


Funding source:None
Conflicts of interest:None
Received on:
December 16, 2016.
Accepted on:
December 23, 2016.