André Correa de Oliveira Romano
DOI: 10.17545/e-oftalmo.cbo/2015.11
ABSTRACT
The rapid development of optical coherence tomography (OCT) and its ophthalmic applications has resulted in new systems that vary in performance and functionality providing in vivo three-dimensional volumetric reconstructions of both anterior and posterior segments of the human eye within unimaginable speed. Newer swept source OCT systems have made it possible to achieve OCT acquisition speeds of several million A-scans/s. Another direction of OCT development includes the introduction of adaptive optics to imaging of the posterior segment of the eye that allows correction of the eye’s static and dynamic aberrations, resulting in the achievement of volumetric cellular resolution retinal imaging. The purpose of this article is to present the various aspects of the development of OCT technology within the context of its ophthalmic applications, as well as, the impact of functional OCT.
Keywords: Tomography, Optical Coherence; OCT, OCT angiography, Optical Coherence Tomography, Angiography, OCTA, En face OCT, Diagnostic Imaging, Imaging of Retina, cornea, glaucoma
RESUMO
O rápido desenvolvimento da tomografia de coerência óptica (OCT) resultou no surgimento de novos sistemas que variam em desempenho e funcionalidade e recriam imagens volumétricas in vivo do segmento anterior e posterior com uma velocidade excepcional. O escaneamento do tecido, à níveis histológicos, em milhões de scans/s através de novos Swept source OCTs, coloca esta tecnologia em patamares inimagináveis. No futuro próximo, a combinação do OCT com óptica adaptiva (AO) permitirá a correção de aberrações estáticas e dinâmicas do olho, possibilitando análise celular retiniana. O objetivo deste artigo é introduzir os vários aspectos do desenvolvimento desta tecnologia dentro do contexto de suas aplicações oftalmológicas, bem como, o impacto de novos OCTs de analise funcional.
Palavras-chave: Tomografia de Coerência Óptica. OCT. Angiografia OCT. Traduzir Optical Coherence. Tomography Angiography. OCTA. En face OCT. Diagnostic Imaging. Imaging of Retina, cornea, glaucoma.
INTRODUCTION
Considering the enormous progress made in recent years, diagnostic imaging in ophthalmology has reached highly sophisticated levels that is indicated by the variety of equipment available and the development of rapid and precise methods for disease diagnosis. This requirement prompted the research of combinations of imaging techniques and has led to collaborations from research groups worldwide in an attempt to meet these requirements.
In this context, optical coherence tomography (OCT), a high-resolution and non-invasive imaging technique that allows the crosssectional imaging of the retina, cornea, and optic nerve, has greatly contributed to this progress.1
The development of this technique over the last decade into one of the most important complementary examinations in ophthalmology is undeniable. With an axial resolution of 5-7 n m, it provides in vivo details of the structures and layers of the retina and other ocular tissues and functions as an optical biopsy method. For these reasons, it has been used in other medical fields, including dermatology, cardiology, oncology, gynecology, and dentistry, among others. Besides the medical field, it has been applied in detecting art forgery by analyzing different layers of paint.
The goal of this study was to assess the impact of OCT in ophthalmology and to explore the recent advances and future perspectives of this technology.
METHODS
HISTORY OF OCT
OCT was developed as part of the doctoral dissertation of David Huang, a PhD student, within the combined program offered by the Harvard University School of Medicine and the Massachusetts Institute of Technology (MIT). This technique was developed in the electrical engineering laboratory led by Huang's adviser, Dr. James Fujimoto. They initially aimed to measure the axial length and corneal thickness but discovered an unprecedented potential for the non-invasive analysis of the retina and other ocular tissues using very high-resolution imaging.1
The experiment was conducted in collaboration with Dr. Joel Schuman, a Harvard ophthalmologist, who worked for countless hours to acquire a single image. To improve imaging speed, Fujimoto recruited Eric Swanson, who at that the time worked with optical communication at MIT's Lincoln Laboratory. The first clinical eye-scanning tests were performed by Dr. Carmen Puliafito’s group, who at the time conducted research at the Massachusetts Eye and Ear Infirmary of Harvard Medical School.

BASIC PRINCIPLES
OCT provides high-resolution imaging based on light rather than sound or radio frequency. A light beam is directed at the target tissue and a small fraction of the light that is reflected below the tissue surface is captured.
It is important to note that most of the light is not reflected but instead is scattered sideways. However, in OCT, optical coherence is used to measure the length traveled by the captured photons. This allows the rejection of most of the photons that dispersed before detection. Accordingly, OCT can acquire three-dimensional (3D) images of dense tissues and reject background signals during the capture of the light that is directly reflected by these tissues.
The technique is based on the analysis and detection of interference signals produced between reference and reflected signals. The system includes an interferometer (known as a Michelson interferometer) with a low-coherence, broad-bandwidth light source. Light is split into the reference and sample ports and then recombined into the detector.
In an OCT system, the light is divided into two arms: the sample (which contains the area of interest) and reference arms (which is usually a mirror).
The combination of the light reflected from the sample and reference arms generates an interference pattern but only if the light from both arms has traveled the same optical distance.
IMAGE FORMATION AND GENERATION
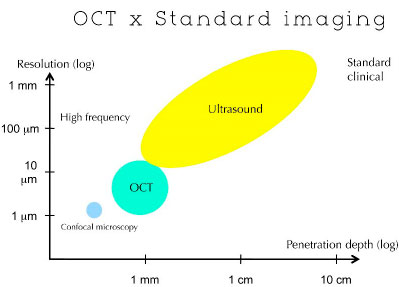
The principles of OCT are similar to those of B-mode ultrasounds and radar because the delay in travel time of reflected photons is used to measure the thickness of the target structure. Unlike ultrasound, OCT can acquire images without coming into contact with the target tissue. (Figure 02)
The most reflective areas of the sample will create more intense interference. Light beams with delayed paths longer than the coherence length will not interfere. The reflectivity profile is referred to as an A-scan and contains information regarding the dimensional and axial position of the structures within the sample. A tomographic image of the cross-sectional section (B-Scan) can be acquired by combining a series of axial scans (A-Scan).2
At a certain depth, en face image generation (C-Scan) allows for variations in the sample position relative to the test beam. In this case, the reflected light is measured at a given depth, the reference mirror remains fixed, and light beams are swept across the sample in successive scans at the x and y coordinates. Therefore, the scanning method employed is similar to that of confocal microscopy.
RESULTS
TYPES OF OCT
Time-Domain OCT (TD-OCT)
The first OCT developed used a time-domain (TD-OCT) system to obtain cross-sectional images of the retina and cornea. This method was present in three generations of retinal OCT (OCT1, OCT3, and Stratus OCT) as well as in the Visante OCT (Carl Zeiss Meditec, Dublin, CA, USA) for the evaluation of the ocular anterior segment.
Using this technique, low-coherence light is produced by a superluminescent diode source coupled to the interferometer. The interferometer contains a beam splitter that separates the light beams into a reference and an imaging beam. The former is directed toward a moving reference mirror, and the latter is directed toward the eye. Both beams are reflected back to the signal detector. The interference pattern created by the two reflected beams generates information on the distance and thickness of the target structures, such as cornea and retina.
The scanning rates in TD-OCT can reach 300 A-scans per second with an axial resolution of 8-10 |jm. At this speed, 3D in vivo images of ocular structures were rare because of physical and technical limitations of the method.
FOURIER-DOMAIN OCT
Two strategies can be used to detect and analyze optical signals using Fourier-domain OCT (FD-OCT): spectral OCT and swept-source OCT. In spectral OCT, a spectrometer and a multichannel analyzer (linear charge-coupled device) are used. In swept-source OCT, a laser source with an optical frequency that rapidly varies is used. (Figure 03)
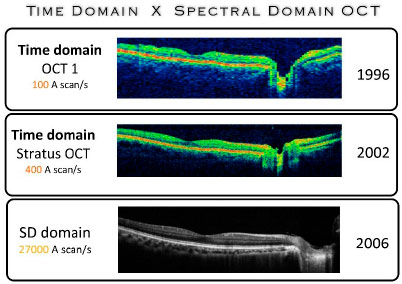
Spectral Domain OCT
Spectral domain OCT (SD-OCT) includes a fixed reference mirror with no mechanical interferences. The interference achieved with the mirror is also fixed. The system uses the detector arm of the interferometer as a spectrometer. The Fourier analysis of the spectral interferogram produces axial scans (A-Scan). Another feature of this system is the scanning of the light source spectrum (swept-source OCT). The scanned images are simultaneously acquired by a camera. Owing to the rapid frame-transfer rate of the camera and to the Fourier analysis algorithm, FD technology increases data acquisition 100-fold without compromising sensitivity. Another advantage was maintaining the system’s sensitivity independent from axial resolution. These resources allow for the acquisition and reconstruction of 3D in vivo ocular images with high axial resolution in a short time frame (seconds or fractions of a second). (Figure 03)

In 2006, the first SD-OCT with axial resolution of 5-7 |jm was introduced in the market, followed by models from many other companies. A major difference observed in this technology was the 100-fold decrease in the time required for tissue scanning, which varies between 20,000 and 52,000 A-scans per second. This ability substantially decreases the number of image artifacts, which is one of the major problems found in previous generations of the equipment, particularly in terms of image reliability and reproducibility. This problem particularly occurred to patients for which the sample fixation was difficult.
Therefore, SD-OCTs initiated the possibility of digitalizing a large retinal area, via volumetric reconstruction, that can be visualized in 3D format with the aid of software. This improvement changed our ability to calculate areas and has allowed the analysis of regions outside the fovea and even the measurement of areas and volumes.
With the incorporation of other technologies, such as color fundus photography, fluorescein angiography, indocyanine green angiography, microperimetry, and autofluorescence, these instruments became known as multimodal imaging. (Figure 4)

SWEPT-SOURCE OCT
The advantages of swept-source OCT (200,000-400,000 Hz) compared with spectral OCT (25,000-70,000 KHz) include improved speed and the ability to acquire images at a wavelength of 1050 nm, resulting in improved image quality in less transparent tissues and consequently, improved visualization of structures, such as the choroid and the optic nerve.3
These novel technologies will have drastic impacts in medicine by reducing the requirement for invasive examinations and will promote the reformulation of clinical concepts and therapeutic protocols, thereby ensuring more personalized medical treatments.
Some OCTs are commercially available, including DRI OCT-1 (Atlantis; Topcon) for imaging of the ocular posterior segment, and OCT SS-1000 (Tomey GmbH, Erlangen, Germany) for imaging of the ocular anterior segment. The former has an acquisition speed of 100,000 A-scans per second that allows easier imaging of larger areas (12x8 mm) in addition to the 3D reconstruction of the entire posterior segment.
These systems can reach speeds of up to 6,700,000 A-scans per second in recently reported research prototypes (Fourier-domain mode-locked laser).4,5
For the first time, speeds of this magnitude may allow real-time 3D video rates for diverse applications, including intraoperative guidance.
RECENT INNOVATIONS
TRACKING AND MOTION-CORRECTION TECHNOLOGY
Several factors, including involuntary eye movements or microsaccades (particularly in patients with fixation issues) may significantly reduce image quality by introducing movement artifacts. The implication of these changes largely depends on the image plane being examined.
One of the solutions incorporated into the current generation of OCTs is the introduction of active eye-tracking technology through either hardware, such as the hardware incorporated in the Spectralis system (Heidelberg) or software, such as those present in Optovue (Fremont, CA, USA) or Carl Zeiss (Dublin, CA, USA) equipment.
However, although the direction of the primary scan creates few movement artifacts, the secondary or slow direction (perpendicular to the direction of the primary scan) results in frequent and significant distortions that prevent reliable analyses of 3D data (3D-OCT).
The possible approaches that can be used to solve this problem include the increase in image acquisition speed5 and its subsequent processing.6
An intelligent alternative that does not depend on the use of high-speed OCT or on subsequent processing is the use of a new algorithm known as motion correction technology (MCT).7
This algorithm relies on the identification of artifacts in a 3D volume in the vertical and horizontal planes, and the two datasets are then combined into a single algorithm. (Figure 05)
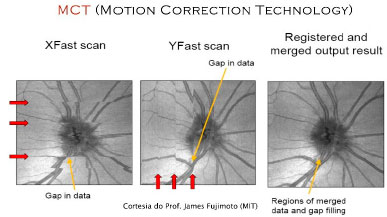
This technology will have a profound effect on en face image analysis as well as on the reliability of blood flow measurements using OCT and angiography.
ENHANCED DEPTH IMAGING and FULL DEPTH IMAGING
At present, two of the most frequently used imaging methods are enhanced depth imaging and deep choroidal imaging.- This technique allows an improved evaluation of the deeper layers of the retina, choroid, and the choroidal-scleral interface. In the SD-OCT systems in use today, essentially two strategies can be used to achieve this result.
The first strategy requires the reduction in speckle noise, which can be achieved using several methods. The most traditional method is image averaging, which involves the calculation of the median of multiple images from the same region and the reduction of the noise level, which can substantially improve image quality.9
Another method involves the modification of the zero-delay line, which is the reference point used by the software to capture images. In addition, this line is standardized at the vitreoretinal junction, resulting in an excellent resolution of the retina. However, protocols, in which the zero-delay line is directed towards deeper layers of the retina, were recently added to the software, ensuring better resolution of the choriocapillaris, Sattler’s layer, Haller’s layer, and the choroidal-scleral interface.8
However, when this modification is combined with other parameters, the visualization from vitreous to sclera can be achieved using a single image without having to make use of an SS-OCT system.
The parameters cited above combined with an increase in the area of axial penetration from 2.3 mm to 3.0 mm and an increase in signal sensitivity would allow for an increase between 500 μ m and 800 μ m, depending on the amount of pigmentation present and on other factors, such as axial length (in cases of myopia). (Figure 06)
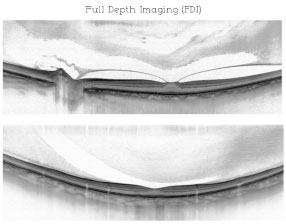
The combination of these techniques results in the acquisition of a single image providing details from vitreous to choroid and is known as full-depth imaging (Romano et al., in press)
EN FACE OCT
Different imaging protocols can be used to generate OCT images, and cross-sectional sections can be acquired using both axial direction (depth) and transverse direction.9
Scanning using both transverse directions allows for en face images at a specific depth. En face imaging is a relatively novel technique from a clinical point of view; however, its capabilities go beyond the traditional methods that are typically used in OCT.10
The largest advantage of en face imaging is that ophthalmologists are familiar with the interpretation of cross-sectional images because their orientations are similar to those produced in fundus photography.
This technique allows the acquisition of 3D images in different planes of motion and identifies the exact location of a diseased tissue. Therefore, it allows a better visualization of the area of interest and the analysis of microstructural changes, and of a more extensive area of the tissue being evaluated. The acquisition of images with this resolution is unfeasible using traditional scanning models.
In addition, this technique allows the identification of subclinical alterations, which may become early markers of disease progression.10,11
OCT Angiography and Doppler OCT
OCT angiography (OCTA) is a novel, non-invasive technique that provides high-resolution angiography images of blood flow within seconds.
To reconstruct the blood flow map, OCTA compares the differences between the intensity and the amplitude of the signal of different B-scans in a specific period.
This technology requires higher scanning speeds that are higher than those currently available in most systems. The capture of each set of 3D scans requires approximately 6 s.
En face images in the angiogram can be acquired from the internal limiting membrane to the choroid. Individual veins and arteries, choriocapillaris, internal and external areas of the retina, and any other areas of interest can be visualized.
The acquired images vary between 2x2 mm and 12x12 mm, and the digitization quality decreases as the visual field increases.
A visual field area of 3 x 3 mm appears to generate better resolution images compared with areas that are currently used in ICG angiography and fluorescein angiography. Matsunaga et al. suggest that this area size could provide a significant amount of detail.12
OCTA demonstrates blood flow at a specific point in time (Figure 7). Though leakages are not always visible, their area and volume can be measured. These images are useful for the analysis of clinical cases, including choroidal neovascularization (CNV) (Figure 08)
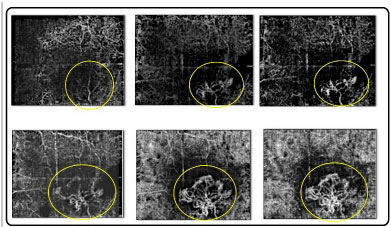
It is particularly useful in the identification of type 1 CNV because of its location and the difficulty in achieving precise access using ICG angiography and fluorescein angiography.
However, the analysis of blood flow may be limited in cases of dense hemorrhaging, in which the access to the retinal layers becomes difficult.
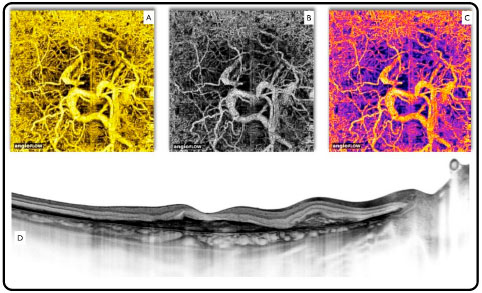
An important advantage of OCTA lies in its ability to simultaneously perform morphological and functional analyses (blood flow). This feature positions this technology on another level when it comes to the evaluation of diseases of the retina and optic nerve, considering that the most common causes of irreversible blindness are associated with abnormal circulation in the optic nerve and macula (glaucoma, diabetic retinopathy, and macular degeneration). In these cases, retinal capillaries can be analyzed in detail and the vascular density of the aforementioned diseased tissues can be evaluated (Figure 9).
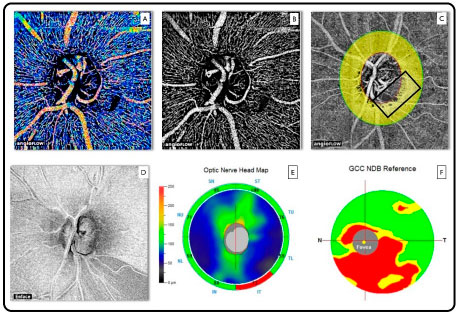
The only OCTA system commercially available to date is the AngioVue software, which uses a specific algorithm known as split-spectrum amplitude decorrelation angiography and is present in the Avanti system (Optovue, Inc., Fremont, CA, USA).
The equipment performs volumetric 304 x 304 A-scans at a speed of 70,000 A-scans per second for approximately 3 s.
The software produces angiograms in a variety of configurations (2x2 mm, 3x3 mm, 6x6 mm, and 8x8 mm) in addition to the automatic segmentation of both the superficial and deep retinal vascular plexuses and the choriocapillaris.
FUTURE PERSPECTIVES
OCT provides images with unprecedented axial resolution. However, their transverse resolution is poor, and as a result, the visualization of retinal cells is unfeasible to date.
The axial resolution in OCT depends on the coherence properties of the light source, which is currently approximately 5 n m and enough to achieve axial resolution in most retinal cells. However, transverse resolution is limited to approximately 15-20 n m in commercial systems.
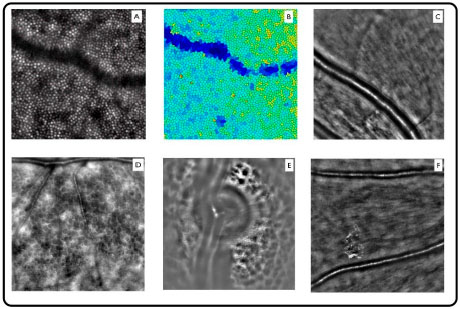
Adaptive optics (AO) was developed to correct these optical aberrations and improve transverse resolution. AO systems measure monochromatic aberrations that occur in the eye and correct them using wavefront sensors and deformable mirrors.
The resolution provided by AO systems allows the acquisition of very high-quality images, including details of photoreceptors, retinal microcirculation, lamina cribrosa, and microstructures, within the nerve fiber and ganglion cell layers (Figure 10).
The first AO applications associated with OCT were recently reported13 and the first human cone images were acquired in 2005.14
An overview of AO-OCT can be found in a recent review.15
AO combined with OCT will drastically change the way ocular diseases are interpreted; however, the use of AO in routine clinical practice is still limited to a small field of vision.
BIOMEDICAL IMAGING, NANOMEDICINE, AND OCT
The use of dyes in ophthalmology was first described in the 1960s as a method for the visualization of retinal and choroidal veins.16 Fluorescein quickly became one of the most important dyes for the identification and classification of a variety of vascular diseases. Although retinal imaging techniques continue to evolve at a rapid pace, considerable shortcomings still exist. However, the combination of technologies, such as OCT, with molecular biomarkers represents a novel strategy on the horizon. Using this approach, receptor-specific exogenous contrast agents can be used to improve OCT capability and allow the visualization of specific cell types and biochemical processes.17
In recent years, the use of nanoparticles has been explored in greater detail.18 Gold nanoparticles are used in CTs to identify tumors.19 In ophthalmology, nanoparticles can be used in conjunction with OCT to monitor the survival of ganglion cells in patients with glaucoma and to evaluate the physiology and changes in the retinal pigmented epithelium (RPE) in different retinal diseases.20
BIOMICROSCOPY VIA OCT
The advent of SD-OCT has created a new perspective of combining OCTs of anterior and posterior segments into a single instrument. It allows the individual analysis of the retina, choroid, optic never, cornea, and anterior chamber angle.
A novel OCT modality known as vertical cavity surface emitting lasers offers the opportunity to increase this ability by capturing the image of the entire eye at once.21
This equipment can capture images with an amplitude of 50 mm and recreate the entire eyeball in a single 3D image.
This ability may represent a novel strategy of ocular evaluation through the introduction of biomicroscopy via OCT and will allow imaging with an unprecedented level of detail and the analysis of pathological features of the cornea, iris, lens (analysis of cataract progression and lens thickness), inflammatory processes in the anterior layer and vitreous, diseases that affect the retina (macula and peripheral retina), choroid, optic nerve, and sclera. This capability will open new horizons for the functional analysis of these structures.
CONCLUSION
Despite the many advances in OCT technology, functional imaging promises to take OCT to another level. Similar to what happened with radiology in the twentieth century, diagnostic imaging in ophthalmology will likely develop into its own specialized field, in which ophthalmologists will become imaging specialists. Novel techniques, including the analysis of blood flow via Doppler OCT and AO-OCT, will change our understanding of disease mechanisms. Therefore, the emerging field of molecular imaging will provide a new perspective into the dynamics of pathological processes in the retina, including inflammation, ischemia, and apoptosis and provide important data on patient susceptibility to disease and disease progression. Furthermore, these techniques will help implement novel therapeutic strategies in daily ophthalmology practice, particularly in the form of ocular biomicroscopy via OCT.
REFERÊNCIAS
1 Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991 Nov 22;254(5035):1178-81.
2 Podoleanu AG, Rosen RB. Combinations of techniques in imaging the retina with high resolution. Prog Retin Eye Res. 2008 Jul;27(4):464-99.
3 Lim H, Mujat M, Kerbage C, Lee EC, Chen Y, Chen TC, de Boer JF. High-speed imaging of human retina in vivo with swept-source optical coherence tomography. Opt Express. 2006 Dec 25;14(26): 12902-8.
4 Klein T, Wieser W, Reznicek L, et al. Multi-MHz retinal OCT. Biomed Opt Express 2013;4:1890-908.
5 T. Klein, W. Wieser, R. Andre, T. Pfeiffer, C. M. Eigenwillig, and R. Huber, “Multi-MHz FDML OCT: snapshot retinal imaging at 6.7 million axial-scans per second,” Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine Xvi 8213(2012).
6 S. Ricco, M. Chen, H. Ishikawa, G. Wollstein, and J. Schuman, “Correcting motion artifacts in retinal spectral domain optical coherence tomography via image registration,” Medical Image Computing and Computer- Assisted Intervention - Miccai 2009, Pt I, Proceedings 5761, 100-107 (2009).
7 Kraus MF, Liu JJ, Schottenhamml J, Chen CL, Budai A, Branchini L, Ko T, Ishikawa H, Wollstein G, Schuman J, Duker JS, Fujimoto JG, Hornegger J. Quantitative SD-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed Opt Express. 2014 Jul 11 ;5(8):2591 -613.
8 Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496-500
9 Hangai M, Yamamoto M, Sakamoto A, Yoshimura N. Ultrahigh-resolution versus speckle noise-reduction in spectral-domain optical coherence tomography. Opt Express. 2009 Mar 2;17(5):4221-35.
10 Romano AC, Belfort. RN., Maia A, Moraes NB, Farah M and Belfort Jr, R. (2009). "En-face, a Novel OCT Imaging Approach to Evaluate Patients With Retinal Diseases." Invest Ophthalmol Vis Sci 50( E-Abstract 341).
11 Lumbroso B, Huang D, Romano A, Coscas G. (2013). "Clinical En Face OCT Atlas." Jaypee Medical Publishers.
12 Matsunaga D, Puliafito CA, Kashani AH. OCT Angiography in Healthy Human Subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):510-5
13 Hermann B, Fern andez EJ, Unterhuber A, et al. Adaptive-optics ultrahigh-resolution optical coherence tomography. Opt Lett 2004;29:2142-4.
14 Zhang Y, Rha J, Jonnal R, et al. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt Express 2005;13:4792-811.
15 Pircher M, Zawadzki R. Combining adaptive optics with optical coherence tomography: unveiling the cellular structure of the human retina in vivo. Expert Rev Ophthalmol 2007;2:1019-35.
16 Novotny HR, Alvis D. A method of photographing fluorescence in circulating blood of the human eye. Tech Doc Rep SAMTDR USAF Sch Aerosp Med. 1960;60-82:1-4.
17 Klein T, Wieser W, Reznicek L, et al. Multi-MHz retinal OCT. Biomed Opt Express [serial online] 2013;4:1890-908.
18 Capozzi ME, Gordon AY, Penn JS, Jayagopal A. Molecular imaging of retinal disease. J Ocul Pharmacol Ther 2013;29: 275-86.
19 Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 2009;38:1759-1782.
20 Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanomedicine in ophthalmology: the new frontier. Am J Ophthalmol 2010;150:144-62.
21 Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012 Nov 1 ;3(11):2733-51.
Fonte de financiamento: declaram não haver.
Conflito de interesses: declaram não haver.
Received on:
December 24, 2014.
Accepted on:
January 30, 2015.