Daniel Caiado Fraga Lavagnoli1; Kássio Assis1; Patrícia Grativol Costa Saraiva1; Fábio Petersen Saraiva1; Luiz Guilherme Marchesi Mello1,2
DOI: 10.17545/eOftalmo/2023.0051
ABSTRACT
Human amniotic membranes have great therapeutic potential owing to their anti-inflammatory, antimicrobial, and antiangiogenic characteristics. In ophthalmology, their use is well established for treating acute and chronic lesions of the ocular surface, such as persistent corneal defects and descemetocele, and even in elective surgeries such as pterygium excision. The process of obtaining amniotic membranes for ophthalmic use involves several steps, including clinically screening the donor, retrieving the placenta along with the amniotic and chorionic membranes, and processing, storing, and monitoring the amniotic membrane quality. Thus, it is necessary to develop a protocol to serve as a guideline for producing amniotic membrane grafts, preserving their therapeutic characteristics and reducing the risks inherent to their use in recipient patients. This study presents a standard operating protocol for procuring, preparing, and preserving amniotic membranes for ophthalmic use to enable the safe reproducibility of this process.
Keywords: Protocols; Tissue banks; Extraembryonic membranes; Tissue and organ procurement; Wounds and injuries.
RESUMO
A membrana amniótica humana possui grande potencial terapêutico por suas características anti-inflamatórias, antimicrobianas e antiangiogênicas. Na Oftalmologia, seu uso está consolidado para o tratamento de lesões agudas e crônicas da superfície ocular, como defeitos corneanos persistentes e descemetocele, e até mesmo em cirurgias eletivas, como a exérese de pterígio. O processo de obtenção da membrana amniótica para uso oftalmológico possui diversas etapas, que incluem a triagem clínica da doadora, captação da placenta com as membranas amniótica e coriônica, processamento, armazenamento e o monitoramento da qualidade da membrana amniótica. Assim, faz-se necessário o desenvolvimento de um protocolo a servir como orientação para a produção de enxertos de membrana amniótica preservando suas características terapêuticas e reduzindo os riscos inerentes ao seu uso em pacientes receptores. Apresentamos neste artigo um protocolo operacional padrão para captação, preparo e preservação da membrana amniótica para uso oftalmológico, de modo a possibilitar a reprodutibilidade segura deste processo.
Palavras-chave: Protocolos, Bancos de Tecidos; Membranas Extraembrionárias; Obtenção de Tecidos e Órgãos; Ferimentos e Lesões.
INTRODUCTION
The placenta is an ephemeral maternal-fetal organ with several functions, including transferring nutrients from mother to fetus and eliminating fetal metabolites. Four different membrane structures are involved in the placental development of human beings: chorion, amnion, yolk sac, and allantois1. During fetal development, the amnion or amniotic membrane (AM) protects the fetus against mechanical stress and dehydration. In addition, AM has other important properties such as anti-inflammatory, antimicrobial, and antiangiogenic activities2. In addition, AM exhibits reduced immunogenicity, contributing to its applicability as a graft.
AM is between 0.02 and 0.5 mm thick and comprises five distinct layers: epithelium, basal membrane, compact layer, fibroblast layer, and spongy layer (Figure 1)3. It has been used for therapeutic purposes for the first time in 1910; however, it was only 30 years later that its use was described in ophthalmology for treating symblepharon2. Currently, there are studies suggesting benefits of using AM in several other ophthalmologic problems, such as burns, Stevens-Johnson syndrome, toxic epidermal necrolysis, corneal perforations and thinning (e.g., descemetocele, neurotrophic ulcers, and epithelial defects), bullous keratopathy, band keratopathy, limbal stem cell deficiency, conjunctival reconstruction, scleritis, grafting for pterygium, ocular surface tumors, and fistulizing surgeries.
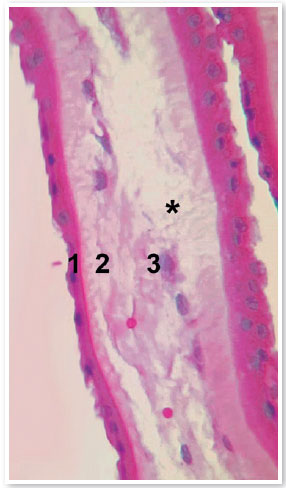
To use AM for ophthalmologic treatment, it is necessary to perform preparation and storage processes for to decontaminating the material and promoting an environment as inert as possible wherein the tissue can be preserved and its properties can be maintained. There are different preservation methods, including dehydration, freeze-drying, chemical crosslinking, and cryopreservation, which can interfere with the tissue structure of AM and influence its degeneration process and treatment success4. A recent study reported that cryopreserved AM is comparable with fresh AM with respect to structural integrity and retention of biochemical components essential for biological functions, indicating that cryopreservation offers a safe and effective means of preservation5. Considering the importance of establishing standardized methods for obtaining AM ready for use in ophthalmic procedures, a standardized operational protocol was developed that establishes flows and methods for procuring, processing, and storing AM.
METHODS
National regulations and international recommendations for using human AM in ophthalmology as well as procuring, preparing, and storing this tissue were reviewed from scientific articles published in PubMed, SciELO, and LILACS bibliography databases in addition to the current Brazilian legislation. Articles published till October 31, 2022 were included, using the following keywords in Portuguese and English: "amniotic membrane," "amnion," "transplantation," "preservation," "tissue bank," and "eye bank." Based on these data, an operational protocol was developed to ensure compliance with good clinical, therapeutic, and organizational practices.
RESULTS
Duties, attributions, and responsibilities
The process of procuring and preparing AM tissue to be suitable for use is multidisciplinary and involves the medical and nursing teams of the ophthalmology clinic's eye bank, obstetrics and gynecology clinic, and pathology and microbiology laboratories, as shown in the flowchart described in Figure 2 and detailed below.
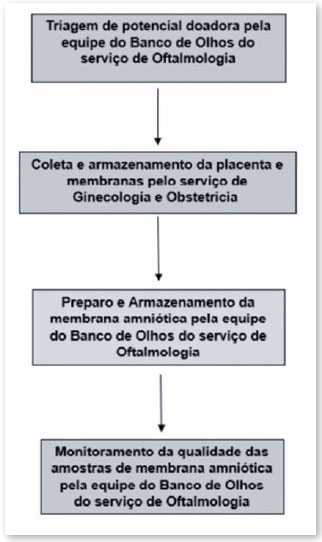
Steps
1. Screening of potential donors by the eye bank team of the ophthalmology clinic
1.1. Identify a possible donor: puerperal women aged >18 years admitted to the obstetric ward (meeting the inclusion criteria) who do not meet any of the exclusion criteria. According to the Technical Regulation of the Brazilian National Transplant System, Ordinance No. 2600 of October 28, 20096-9 and the criteria of the Eye Bank of the Cassiano Antônio Moraes University Hospital of the Federal University of Espírito Santo, Vitória, Brazil, the absolute contraindications to a candidate for donation were defined as follows:
a) Active sepsis;
b) Active endocarditis (bacterial or fungal);
c) Active disseminated lymphomas;
d) Leukemias;
e) Clinical or laboratory evidence of infection caused by the human immunodeficiency virus (HIV) or hepatitis B or C;
f) Risk of transmission of diseases caused by prions (such as Creutzfeldt-Jakob disease), neurological diseases of viral or undetermined etiology, subacute sclerosing panencephalitis, active viral encephalitis, encephalitis of unknown origin, progressive encephalopathy, or progressive multifocal leukoencephalopathy;
g) Rabies;
h) Congenital rubella;
i) Reye's syndrome;
j) Meningitis;
k) Colonization by multidrug-resistant bacteria;
l) Pesticide poisoning;
m) Leptospirosis;
n) Dengue fever in the febrile or critical phase;
o) Malaria;
p) Herpetic encephalitis;
q) Cancer in a location more likely to metastasize (breast, lung, liver, pancreas, lymphomas, leukemias, and cutaneous melanomas);
r) The following active diseases: cancer of the uterus, cervix, or vaginal canal; endometriosis; endometritis; pelvic inflammatory disease; and intrauterine or vaginal canal infection with the human papillomavirus (HPV).
1.2 Approach and reception of the pregnant woman for the placenta donation process.
This is a vital step and the interview needs to be conducted responsibly, respectfully, and carefully, especially because the questionnaire presented to the pregnant woman includes personal and intimate questions about their behavior, and therefore, requires a comfortable environment to be answered.
Deliver the Placental Tissue Donation Term (Figure 3), collecting signatures in all three copies. The first copy will be attached to the medical record, the second copy will be attached to the donation record at the eye bank, and the third copy will be provided to the potential donor. Refusal to sign the Placental Tissue Donation Term will be considered an exclusion criterion.
1.3. Identify the initial data of the AM donor (Figure 4), including name, age, time of placental expulsion, medical record number, and family members present;
1.4. Fill out the clinical screening form (Figure 5). Failure to meet all mandatory criteria will make it impossible to use the donated tissue, and it must be disposed of in an appropriate place:
a) Delivery <24 h before the day the graft is made (mandatory criterion);
b) No history of alcohol, tobacco, or drug abuse (mandatory criterion);
c) No history of multiple partners in the last 9 months (mandatory criterion);
d) Evaluation of prenatal screening exams (pregnant woman's card). A positive result in any of the following tests is a criterion for exclusion from this protocol (mandatory criterion): HBsAg, anti-HBc (IgM and IgG), anti-HCV, anti-HIV-1, anti-HIV-2, VDRL, HTLV-1 and -2, CMV (IgM and IgG), toxoplasmosis (IgM and IgG);
e) Preferentially, delivery at term with no confirmed or suspected associated comorbidities such as placental abruption, intrauterine growth restriction, malformations, venereal or congenital infections, meconium during childbirth, acute or chronic fetal distress, premature rupture of membranes, or endometritis (optional criterion).
Collect blood samples from the donor and fill out the laboratory screening form (Figure 4). Positivity for one (or more) of the tests will make it impossible to use the donated tissue, and it should be discarded in an appropriate place.
2. Collection and storage of placenta and membranes by the gynecology and obstetrics clinic
• Hand hygiene according to the standard operating procedure of the Hospital Infection Control Department;
• After placental expulsion, while properly fitted with personal protective equipment (goggles, mask, and sterile glove), place the potential donor's placenta and membranes in a collection bag;
• Close and identify the collection bag with the parturient's full name and medical record number;
• Store the collection bag for up to 24 h (from the time of delivery) in an environment with controlled temperature between 2ºC and 8ºC;
• Discard in an appropriate place any collection bag that has been stored for >24 h.
3. Preparation of the amniotic membrane by the eye bank team of the ophthalmology clinic
3.1. After identifying a donor, completing all documentation, and obtaining authorization for using AM, transport the properly labeled collection bag containing the placenta and its membranes directly to the eye bank in an isothermal box at an internal temperature of 2ºC to 8ºC.
3.2. Separation and initial preparation of the amniotic membrane
• Hand hygiene according to the institution's protocol;
• Dress with personal protective equipment (goggles, cap, mask, sterile gown, and sterile glove) and distribute the sterile drapes in the work area;
• Separate AM and chorionic membrane from the rest of the placenta using sterile scissors, keeping the epithelial surface of the AM (inner side of the membrane) always facing upwards;
• Return the rest of the placenta to the collection bag;
• Wash the AM and chorion thoroughly with using Ringer's lactate solution;
• Manually separate the chorionic membrane from AM and place the chorion together with the rest of the placenta in a collection bag for proper disposal;
• Arrange two vats and fill them with solution 1 (prepared according to the supplementary file Description of solutions 1 and 2);
• AM is divided into two parts, each of which must be placed in a sterile vat or basin and irrigated abundantly with sterile Ringer's lactate for manual and mechanical removal of blood and debris, changing the Ringer's lactate according to need;
• Place the clean membranes in the vats containing solution 1 (Figure 6), and wait 30 min;
• Remove the gloves and sterile gown;
• Sanitize your hands.
3.3. Final preparation of the amniotic membrane samples in a laminar flow hood for storage
• Sanitize hands according to the institution's standard protocol;
• Sanitize the laminar flow hood before handling it according to the institution's standard protocol;
• Arrange the sterile vials for AM storage (Figure 7), according to the mode of preservation to be used in the membranes;
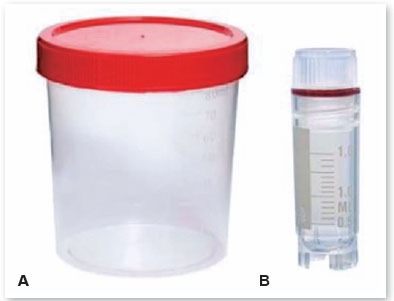
(NOTE: The sterile vial must be selected according to the temperature of the place where it will be preserved. Those intended for temperatures from 2ºC to 8ºC are made of polypropylene or polyethylene and have a single screw cap, while those made to withstand cold temperatures up to -85ºC are made of reinforced polypropylene, with an attached lid, hermetic closure, and double seal.)
• Hand hygiene according to the institution's protocol;
• Dress with personal protective equipment, sterile gown, and sterile glove and distribute the sterile drapes in the work area in the laminar flow hood;
• Introduce the AM storage vials into the laminar flow hood, open them, and place them in the sterile area;
• Arrange the sterile material and the other consumables to be used on the hood bench and open it in such a way as not to contaminate it with the help of an assistant who is not dressed in sterile clothing;
• Arrange nitrocellulose paper, tweezers, scissors, and scalpel on the sterile impermeable drapes;
• Place AM on the nitrocellulose paper with the epithelial surface facing upwards;
• Start cutting the AM in 2.5 × 2.5-cm pieces (Figure 8) and place each piece on nitrocellulose paper in a storage vial containing 10 mL solution 2 (prepared according to the supplementary file Description of solutions 1 and 2);

• Place a piece of AM in a tube containing brain-heart infusion (BHI) and send it to the microbiology department (for investigation of fungi, bacteria, nonspecific germs, bacterioscopy, and antibiotic susceptibility testing), incubating for up to 15 days;
• Send a vial containing an AM sample along with the collection bag containing the placenta and chorionic membrane for analysis in the pathology department;
• Discard the blades in the specific box for sharp objects;
• Remove the materials from the hood after the end of the procedure and send them for disposal;
• Remove the personal protective equipment;
• Sanitize hands according to the institution's standard protocol;
• Put on procedure gloves;
• Label the AM storage vials with the identifications of the product (amniotic membrane and size), number of the donation record in the eye bank, name of the person responsible for the preparation, storage temperature, date of preparation, expiration date of the amniotic membrane (2ºC to 8ºC: 3 months; -85ºC to -75ºC: 2 years,10,11) and seal the vials;
• Keep the jars in the refrigerator (2ºC to 8ºC) or freezer (-80ºC).
4. Monitoring the quality of the amniotic membrane samples by the eye bank team of the ophthalmology clinic
Following the preparation and storage of the AM samples, monthly monitoring of the batches is required through external visual inspection and by sending a sample for microbiological and histopathological analyses. Thus, the material will only continue to be released for use if it does not present alterations on external visual inspection, signs of infection on microbiological and histopathological examination, or signs of advanced tissue necrosis on histopathological examination. The results of these analyses must be stored in the donation record at the eye bank by filling out the sample quality monitoring form (Figure 9).
If any problem is detected with a membrane supplied for use, the physician who used it must notify the eye bank in writing about the problem, and the supply of samples from that batch will be suspended until the situation is investigated with the technician responsible for the eye bank, who will decide whether the samples will be discarded or kept for use. The data must be annotated in the eye bank's donation record.
DISCUSSION
The reality of ophthalmological services in Brazil with regard to the use of AM is still limited to large research centers and universities. Part of this scenario is because of the hospital and laboratory structure necessary to enable the procurement, processing, storage, monitoring, and use of the material in patients with this indication. In view of this, it is extremely important that resolutions such as the one promulgated by the Brazilian National Health Surveillance Agency, Collegiate Board Resolution No. 55 of December 11, 20158, be formulated with the aim of standardizing tissue banks. Thus, minimum technical and sanitary requirements should be established for the operation of these facilities, aiming at the safety and quality of the tissues supplied for therapeutic use8, to enable new application centers to appear and more people to take advantage of this resource.
It is obvious that a process with so many steps needs a cohesive multidisciplinary team comprising trained and qualified professionals who will work from the moment of clinical and laboratory screening of the pregnant woman to the monitoring of the sample to enable the use of the material4. In addition to the human resources in this process, it is equally essential for users to have access to an adequate structure for the correct storage of the material, laboratory for histopathological analysis, and laboratory for the detection of possible microorganisms contaminating the membrane.
This way it should be possible to produce a surgical graft with good structural quality and little risk of contamination to be used for the benefit of patients with ophthalmic diseases. In addition, the preservation and long-term storage of biological materials not only offers the advantage of good availability for a longer period but it also reduces the risk of disease transmission10,11.
The use of AM in ophthalmology is one of the main therapeutic options for ocular surface diseases refractory to clinical treatment, especially those involving alterations in the healing process and the ocular physical barrier. To date, no articles have been published in the scientific literature to guide the collection, preparation, storage, and monitoring of human AM samples according to Brazilian and international quality standards. The development of a clinical protocol as described in this article is essential to ensure the quality of AM samples, reduce risks to patients, and popularize the use of this tissue in daily ophthalmological practice.
REFERENCES
1. Brolio MP, Ambrósio CE, Franciolli AR, Morini AC, Guerra RR, Miglino MA. A barreira placentária e sua função de transferência nutricional. Rev Bras Reprod Anim. 2010;34(4):222-232.
2. Alió JL, Abad M, Scorsetti DH. Preparation, indications and results of human amniotic membrane transplantation for ocular surface disorders. Expert Rev Med Devices. 2005;2(2):153-60.
3. Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67(5):662-75.
4. 4Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193-204.
5. Hettiarachchi D, Dissanayake VH, Goonasekera HW. Optimizing amniotic membrane tissue banking protocols for ophthalmic use. Cell Tissue Bank. 2016;17(3):387-97.
6. Saúde Md. PORTARIA Nº 2.600, DE 21 DE OUTUBRO DE 2009 Aprova o Regulamento Técnico do Sistema Nacional de Transplantes. In: Ministro Gd, editor. Diário Oficial da União 2009.
7. Saúde Md. PORTARIA DE CONSOLIDAÇÃO Nº 4, DE 28 DE SETEMBRO DE 2017 Consolidação das normas sobre os sistemas e os subsistemas do Sistema Único de Saúde. In: Ministro Gd, editor. Diário Oficial da União 2017.
8. Colegiada D. RESOLUÇÃO DA DIRETORIA COLEGIADA - RDC Nº 55, DE 11 DE DEZEMBRO DE 2015. In: Sanitária ANdV, editor. Diário Oficial da União 2015.
9. Saúde Md. RESOLUÇÃO Nº 67, DE 30 DE SETEMBRO DE 2008. In: Sanitária ANdV, editor. Diário Oficial da União 2008.
10. Maral T, Borman H, Arslan H, Demirhan B, Akinbingol G, Haberal M. Effectiveness of human amnion preserved long-term in glycerol as a temporary biological dressing. Burns. 1999;25(7):625-35.
11. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51-77.
| AUTHORS INFORMATION |
|
 |
» Daniel Caiado Fraga Lavagnoli https://orcid.org/0000-0002-2783-3753 http://lattes.cnpq.br/7138201543100306 |
 |
» Kássio Assis https://orcid.org/0000-0002-0226-1816 http://lattes.cnpq.br/9733413369910165 |
 |
» Luiz Guilherme Marchesi Mello https://orcid.org/0000-0001-8347-2393 http://lattes.cnpq.br/0620671396588526 |
 |
» Patrícia Grativol Costa Saraiva https://orcid.org/0000-0002-1083-9980 http://lattes.cnpq.br/8944400266170157 |
 |
» Fábio Petersen Saraiva https://orcid.org/0000-0002-1196-8872 http://lattes.cnpq.br/4779368875052793 |
Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
May 17, 2023.
Accepted on:
October 22, 2023.