Thaissa Faloppa Duarte; Rubens Camargo Siqueira
DOI: 10.17545/eOftalmo/2023.0025
ABSTRACT
Meibomian gland dysfunction is a chronic, multifactorial abnormality characterized by obstruction of the terminal duct and/or qualitative and quantitative changes in glandular secretion that results in a change in the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease and can cause vision impairment, reducing quality of life. Ophthalmological, systemic and medication-related factors can coexist or contribute to the pathogenesis of this dysfunction, contributing to the prognosis and stabilization of the disease. Diagnosis and treatment are essentially clinical. The present review article describes clinical and diagnostic aspects of meibomian gland dysfunction.
Keywords: Meibomian gland dysfunction; Systemic diseases; Ophthalmology.
RESUMO
A disfunção da glândula meibomiana é uma anormalidade crônica caracterizada por obstrução do ducto terminal e/ou alterações qualitativas e quantitativas na secreção glandular, podendo resultar em alteração do filme lacrimal, sintomas de irritação ocular, inflamação clinicamente aparente e doença da superfície ocular, que podem acarretar grandes prejuízos visuais e de qualidade de vida. Fatores oftalmológicos, sistêmicos e relacionados com medicações podem coexistir ou contribuir para a patogênese dessa disfunção, contribuindo no prognóstico e estabilização da doença. Seu diagnóstico e tratamento são essencialmente clínicos. Neste artigo de revisão são descritos aspectos clínicos e diagnósticos de pacientes com disfunção da glândula meibomiana.
Palavras-chave: Disfunção da glândula meibomiana; Doenças sistêmicas; Oftalmologia.
INTRODUCTION
Meibomian gland dysfunction (MGD) is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal-duct obstruction, qualitative and quantitative changes in glandular secretion, or both. It can result in an altered tear film, ocular irritation symptoms, clinically apparent inflammation, and ocular-surface disease1.
Described by German physician Heinrich Meibom in 1666, the meibomian glands are modified sebaceous glands located in the tarsal plates of the upper and lower eyelids. These glands actively synthesize and produce lipids and proteins that are spread on the tear film, promoting its stability by decreasing surface tension and preventing evaporation of the aqueous phase. Each meibomian gland consists of several se-cretory acini containing meibocytes, lateral ductules, a central duct, and a terminal excretory duct that opens to the posterior margin of the eyelid. Its secretion (meibum) is released into the eyelid through muscle contractions during eyelid movement. These glands are densely innervated and their function is regulated by androgens, estrogens, progesterone, retinoic acid, growth factors, and, probably, neurotransmitters1,2.
This review article presents the clinical aspects and diagnosis of MGD. In addition, a multidisciplinary therapeutic approach to the occurrence of this dysfunction in patients with systemic diseases is discussed.
Epidemiology
The incidence of MGD ranges from 3.5% to 70%3-15, being high in Asian populations (46.2 to 69.3%), particularly in men4-6. By contrast, in Caucasians it ranges from 3.5% to 19.9% and is more common in women.(3) The consensus is that its frequency increases with age. In Brazil, MGD has an incidence between 37.0% and 50.0%, and it more frequently affects women and individuals aged >50 years16–18.
Etiology
MGD can be divided into the following two categories based on the secretion by the meibomian glands: low- and high-secretion forms. Low-secretion forms are classified as obstructive hyposecretory and can be cicatricial or non-cicatricial17–19. In hyposecretory MGD, the reduced secretion is caused by abnormalities in the meibomian glands themselves without significant obstruction. It is usually caused by an obstruction of the terminal duct. In the cicatricial form, the duct ostia are dislodged posteriorly into the mucosa, an occurrence known as the conjunctivalization of the eyelid margin. This is important for detecting the cicatricial type of chronic meibomitis, as the ostia remain in their normal position in non-cicatricial MGD.
Histopathological changes include hypertrophy and keratinization of the epithelium of the ostium. Low secretion is caused by glandular obstruction associated with terminal-duct obstruction and consequent secretion stasis, which result in an intraglandular cystic dilatation, acinar atrophy, and consequently decreased lipid secretion. This disorder is observed in elderly individuals as a result of toxicity from topical and systemic medications, such as the use of systemic retinoic acid and chronic use of contact lenses1,2. Androgen insufficiency or lack of androgen receptors is also associated with keratinization, obstruction, and altered secretions of the meibomian glands. Causes of cicatricial obstructive MGD include trachoma, ocular cicatricial pemphigoid, erythema multiforme, and atopic eye disease. Non-cicatricial obstructive MGD can be seen in Sjögren’s syndrome, seborrheic dermatitis, acne rosacea, atopy, and psoriasis. It is well known that commensal bacteria such as coagulase-negative staphylococci, Staphylococcus aureus, and Propionibacterium acnes contribute to the clinical worsening of the disease, as bacterial enzymes lead to the release of lipid degradation products such as free fatty acids, which activate the inflammatory cascade, thereby exacerbating keratinization.
Hypersecretory MGD is characterized by the release of a large volume of meibomian lipids on the eyelid margins in response to pressure on the tarsus. This pressure can be primary (idiopathic) or secondary to dermatological diseases, such as seborrheic dermatitis, atopic dermatitis, and acne rosacea1,2,20–24.
As a result, there is increased tear evaporation, hyperosmolarity, instability, and deficiency of wetting, leading to mechanical irritation that stimulates inflammatory cascades on the ocular surface and bacterial proliferation on the eyelid margin. This intensifies the inflammation of the ocular surface6,18,25–27.
Risk Factors
There are several ophthalmological, systemic, and medication-related factors that may coexist or contribute to the pathogenesis of MGD.
Ophthalmological factors include chronic contact lens use, Demodex folliculorum infestation, floppy eyelid syndrome, giant papillary conjunctivitis, ichthyosis, Salzmann’s corneal degeneration, trachoma, and dry-eye disease due to tear-film deficiency2,3,15,28,29.
Systemic factors that may favor MGD include androgen deficiency, menopause, aging, Sjögren’s syndrome, cicatricial pemphigus, Stevens-Johnson syndrome, systemic lupus erythematosus, hypercholesterolemia, psoriasis, atopy, rosacea, systemic arterial hypertension, benign prostatic hyperplasia (BPH), allogeneic or autologous stem-cell transplantation, ectodermal dysplasia syndrome, Parkinson’s disease, polycystic ovary syndrome, toxic epidermal necrolysis, and Turner’s syndrome1,3–13,22,24,25,30.
Medications associated with the pathogenesis of MGD include antiandrogens, medications for BPH, hormone therapy (estrogens and progestins), antihistamines, antidepressants, and systemic retinoids (isotretinoin)31.
Environmental factors such as geography, temperature, humidity, and visual tasks may play a role in MGD or have an impact in worsening symptoms in patients, by aggravating tear-film dysfunction. The increased frequency of MGD in Asian populations may be related to geographical climate differences (temperature, humidity, and air quality)3,27. Computer users (who have decreased blinking frequency) often complain of eye fatigue, burning sensation, irritation, and redness.
Clinical Features and Diagnosis
Clinically, MGD can be categorized into the following four subtypes: 1. Primary MGD, which is subdivided into asymptomatic and symptomatic, and the latter in turn is subdivided into cicatricial and non-cicatricial; 2. MGD associated with damage to the ocular surface; 3. MGD-related evaporative dry eye; and 4. MGD related to other ocular disorders.
The International Workshop on Meibomian Gland Dysfunction Report suggests a severity classification into 5 levels, described below, based on an assessment of the symptoms (frequency and severity, and Ocular Surface Disease Index-OSDI grading) and of meibomian gland function (eyelid morphology, gland atrophy, meibum expressibility, and secretion quality)1,32,33.
Level 1: Subclinical: absence of ocular discomfort symptoms, itching, or photophobia, in the presence of clinical signs of MGD based on gland expression (minimally altered secretions), without discoloration of the ocular surface;
Level 2: Minimal symptomatic: symptoms of ocular discomfort, itching, or photophobia, associated with clinical signs of MGD based on gland expression (minimally altered secretions), without discoloration of the ocular surface;
Level 3: Mild symptomatic: symptoms of ocular discomfort, itching, or photophobia, associated with minimal to mild clinical signs of MGD (slightly altered secretions), without discoloration of the ocular surface (Figure 1);

Level 4: Moderate symptomatic: moderate symptoms of ocular discomfort, itching, or photophobia, with limitation of activities, associated with moderate clinical signs of MGD (obstruction, vascularity, moderately altered secretions, and mild to moderate discoloration of the peripheral cornea and conjunctiva, often in an inferior location) (Figure 2);
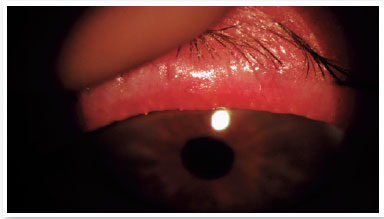
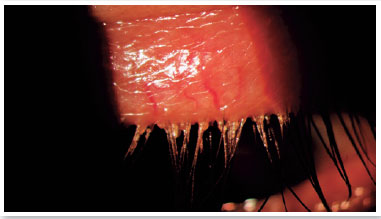
Level 5: Severe symptomatic: frequent and intense symptoms of ocular discomfort, itching, or photo-phobia, with limitation of activities, associated with severe clinical signs of MGD (severely altered secretions and increased discoloration intensity), and conjunctival and corneal inflammation, including in the central region (Figure 3).
MGD is often asymptomatic; however, it may be associated with several symptoms including itching, burning in the eyes, sandy feeling in the eyes, swollen eyelids, eye dryness, eye irritation, lacrimation, eyelash crusting (especially in the morning), gluey eyelids, eye redness, mild photophobia, visual blurriness, burning sensation, hyperemia of the eyelid margins, and crust formation, among others17,34–36. Symptoms can vary in frequency and severity, and can be influenced by climate, humidity, or activity level (screen use). Understanding and quantifying these subjective aspects of MGD and its perceived impact on the individual’s life are required for valid techniques to be used to assess MGD3.
The purpose of the slit-lamp ophthalmological examination is to identify morphological changes in the eyelid margins, such as changes in vascularization, telangiectasia, ulcers, keratinization, scales, and crusts-also known as eyelash collarettes. The eyelid margin may be thickened and have a cutaneomucosal junction (Marx’s line), which may be internalized. The examiner must observe whether the drainage ostia of the meibomian glands are well delimited and surrounded by regular epithelium. Large, enlarged, fat-covered ostia indicate chronic inflammation. Non-visible ostia are the result of non-functioning glands, with an absence of secretion and the presence of keratin and desquamated epithelial cells at the drainage site17,36. The clinical picture may also include hordeola, chalazion, absence of eyelashes (madarosis), or poor positioning of the eyelashes (trichiasis and distichiasis)3.
Following ophthalmological examination of the cornea, conjunctiva, and tear film, the tarsal conjunctiva can manifest hyperemia, inflammation, papillary reaction, and scarring. The bulbar conjunctiva may show hyperemia and phlyctenules. The cornea and the conjunctiva may show superficial punctate keratopathy, phlyctenulosis, and marginal infiltrates in areas that correspond to the touch points between the cornea and the eyelid18.
For patients with ocular surface symptoms or morphological signs of MGD on the eyelid, meibomian gland function should be assessed by finger pressure on the central third (slightly nasally) of the lower and upper eyelids to determine the extent and severity of MGD (ease of expulsion and secretion quality).
Partial or total meibomian gland atrophy can be quantified using meiboscopy, meibography, and confocal microscopy.
The patient should be assessed for evidence of ocular surface damage and dry eyes using specific tests, including measurement of tear osmolarity, tear secretion testing (fluorophotometry or fluorescein clearance rate), measurement of tear volume in the eye (fluorophotometry and meniscometry), stability testing (tear breakup time or interferometry), and measurement of tear evaporation (evaporimetry). Tests for damage to the ocular surface, including staining of the cornea and conjunctiva, are also included in the test series. Evaluation of inflammatory mediators, for the presence of inflammatory cell markers, and other proteomic and lipidomic mass spectrometry analyses can provide information about the overall inflammatory status of the ocular surface. Specific tear production measurements for the diagnosis of aqueous deficient dry eye, such as the Schirmer test, are also recommended.
TREATMENT
The International Workshop on Meibomian Gland Dysfunction Report standardized the treatment according to the stage of the disease, according to the clinical findings:
Level 1: patient orientation on diet, medications, and work or home environments that worsen dryness, associated eyelid hygiene, and warm compresses;
Level 2: level 1 guidelines associated with improved humidification of the environment, increased dietary intake of omega3 fatty acids, eyelid hygiene with warm compresses (minimum 4 minutes, once or twice a day), artificial lubricants (frequent use, preferably preservative-free), topical or liposomal-spray emollient lubricant, and topical azithromycin or oral tetracycline.
Levels 3 and 4: all of the items in level 2 coupled with oral tetracycline, emollient lubricating ointment at bedtime, and anti-inflammatory therapy for dry eyes25.
All diagnosed patients, regardless of stage, benefit from systematic and continuous hygiene of the eyelid margin. Increasing the local temperature with warm compresses and massage facilitates secretion flow, duct clearance, and vascular circulation around the glands1,14,17,26,36. Alternative heat sources for therapy with warm compresses include ocular warming devices, infrared irradiation, moist air, and ocular warming masks1.
In cases of chronic local bacterial infection, topical antibiotic ophthalmic ointments can be prescribed one to four times a day, depending on the severity of the process, for one to four weeks.
Topical application of corticosteroid ophthalmic ointments on the eyelid margin may be considered with the aim to suppress acute inflammation and associated allergic processes. Their use should be short-term because of the risk of increased intraocular pressure and cataract induction. Cyclosporine and tacrolimus eye drops have been used in clinical practice as anti-inflammatory alternatives to corticosteroids.
Oral antibiotics such as tetracycline, doxycycline, or azithromycin modify the local microbiota and improve lipid secretion by directly reducing the production of bacterial lipases by Staphylococcus aureus, Staphylococcus epidermidis, and Propionibacterium acnes, decreasing the concentration of free fatty acids and their harmful effects on the lipid layer of the tearfilm1,14,17,26.
Ocular lubricants are an important support to tear dysfunction management, since MGD is often associated with tear-film dysfunction.
FINAL REMARKS
In clinical practice, ophthalmological impairment caused by MGD may be observed, and so can the impact of this disease on the individual’s quality of life. Owing to its high prevalence, its socioeconomic impact is significant.
Because MGD is a chronic disease, follow-up must be periodic, with a multidisciplinary approach, considering other associated diseases, medications used by the patient, and even environmental factors.
Therefore, knowledge related to MGD from scientific studies is necessary not only for ophthalmologists but also for the entire medical community, thereby highlighting the importance of the individual being considered holistically.
REFERENCES
1. MGD redefined: International Workshop on Meibomian gland dysfunction report. Boston: The Tear Film & Ocular Surface Society (TFOS); 2011. Disponível em: www.tearfilm.org/mgdworkshop/index.html.
2. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-78.
3. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005.
4. Chen A, Chen HT, Chen HC, Chen YT, Hwang YH, Sun CC, et al. Asymptomatic meibomian gland dysfunction and cardiovascular disease risk factors in a middle-aged population in Taiwan: a cross-sectional analysis. Sci Rep. 2017;7(1):4935.
5. Siak JJ, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, et al. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea. 2012;31(11):1223-8.
6. Tulsyan N, Gupta N, Agrawal N. Risk factors associated with meibomian gland dysfunction: a hospital based study. Nepal J Ophthalmol. 2021;13(25):59-64.
7. Yamaguchi M, Nishijima T, Shimazaki J, Takamura E, Yokoi N, Watanabe H, et al. Real-world assessment of diquafosol in dry eye patients with risk factors such as contact lens, meibomian gland dysfunction, and conjunctivochalasis: subgroup analysis from a prospective observational study. Clin Ophthalmol. 2015 Dec 1;9:2251-6.
8. Braich PS, Howard MK, Singh JS. Dyslipidemia and its association with meibomian gland dysfunction. IntOphthalmol. 2016;36(4):469-76.
9. Dao AH, Spindle JD, Harp BA, Jacob A, Chuang AZ, Yee RW. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am J Ophthalmol. 2010;150(3):371-5.e1.
10. Kuriakose RK, Braich PS. Dyslipidemia and its association with meibomian gland dysfunction: a systematic review. Int Ophthalmol. 2018;38(4):1809-16.
11. Guliani BP, Bhalla A, Naik MP. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J Ophthalmol. 2018;66(10):1411-6.
12. Mussi N, Haque W, Robertson DM. The association between risk factors for metabolic syndrome and meibomian gland disease in a dry eye cohort. Clin Ophthalmol. 2021 Sep 11;15:3821-32.
13. Lin X, Wu Y, Chen Y, Zhao Y, Xiang L, Dai Q, et al. Characterization of meibomian gland atrophy and the potential risk factors for middle aged to elderly patients with cataracts. Transl Vis Sci Technol. 2020;9(7):48.
14.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 Suppl):S1-14.
15.Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci. 1996;73(3):208-10.
16.Adam Netto AA, Rolim AP, Muller TP. Prevalência de doenças palpebrais no serviço emergencial de oftalmologia do hospital universitário da Universidade Federal de Santa Catarina. ACM arq catarin med. 2006;35(4):64-9.
17. Santo RM. Blefarite e blefaroconjuntivitivites. In: Alves MR, Nakashima Y, Tanaka T, editores. Clínica oftalmológica: condutas práticas em oftalmologia. Rio de Janeiro: Guanabara Koogan; 2013. p. 436-7.
18. Hida RY, Santo RM. Blefarites e disfunção das glândulas de Meibomius. In: Alves MR, Santo RM, Sousa SJF, Costa DC, editores. Essencial em blefaroconjuntivites. São Paulo: Omnifarma; 2012. p. 13-24.
19. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):163-78.
20. Motta AA, Aun MV, Kalil J, Giavina-Bianchi P. Dermatite de contato. Rev Bras Alerg Imunopatol. 2011;34(3):73-82.
21. Leung DM, Eichenfield LF, Boguniewicz M. Atopic dermatitis (atopic eczema). In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editores. Fitzpatrick’s dermatology in general medicine. New York: McGraw Hill; 2008. p.146-58.
22. Hanifin JM, Rajka G. Diagnostic features of atopic dermatites. Acta Dermatovener (Stockholm). 1980;92(Suppl):44-7.
23. Ferolla C. Dermatite seborreica da face.Rev Bras Med. 2010;67(supl 9):11-5.
24. Al Arfaj K, Al Zamil W. Spontaneous corneal perforation in ocular rosacea. Middle East Afr J Ophthalmol. 2010;17(2):186-8.
25. Vieira AC, Höfling-Lima AL, Mannis MJ. Ocular rosacea: a review. ArqBras Oftalmol. 2012;75(5):363-9.
26. Adán CBD, Araujo MERX, Hofling-Lima AL, Nishiwaki-Dantas MC, Alves MR. Doenças externas oculares e córnea. Série Brasileira de Oftalmologia, CBO. 3ª ed. Rio de Janeiro: Cultura Médica, Gen, Guanabara Koogan; 2013.
27. Asbell PA, Stapleton FJ, Wickström K, Akpek EK, Aragona P, Dana R, et al. The international workshop on meibomian gland dysfunction: report of the clinical trials subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2065-85.
28. Dias MR, Guaresch BLV, Borges CR, Biazim DF, Casagrande D, Luz RA. Blefarite: epidemiologia, etiologia, apresentações clínicas, tratamento e evolução de nossos pacientes. Rev Bras Oftalmol. 2019;78(5):300-3.
29. Holzchuh FG, Hida RY, Moscovici BK, Villa Albers MB, Santo RM, Kara-José N, et al. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am J Ophthalmol. 2011; 151(6):1030-4.e1.
30. Sullivan DA, Sullivan BD, Evans JE, Schirra F, Yamagami H, Liu M, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002 Jun;966:211-22.
31. Baser G, Yildiz N, Calan M. Evaluation of meibomian gland dysfunction in polycystic ovary syndrome and obesity. Curr Eye Res. 2017;42(5):661-5.
32. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108-52.
33. Design and conduct of clinical trials: report of the Clinical Trials Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):153-62.
34. Acharya N, Pineda R 2nd, Uy HS, Foster CS. Discoid lupus erythematosus masquerading as chronic blepharoconjunctivitis. Ophthalmology. 2005;112(5):e19-23.
35. Xavier ME, Souza MB, Hofling-Lima AL, Santo RM, Alves MR. Blepharitis, conjunctivitis and trachoma. In: Boyd BF, editor. Modern ophthalmology: the highlights the account of a master witnessing a 60 epoch of Evolution and progress (1950-2010). Panama: Jaypee-Highlights Medical Publishers; 2010. p. 221-240.
36. Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-64.
AUTHOR’S INFORMATION


Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
December 15, 2022.
Accepted on:
March 13, 2023.