Bruno Hirt1; Carlos Augusto Moreira-Neto1,2
DOI: 10.17545/eOftalmo/2023.0018
ABSTRACT
COVID-19 infection leads to a state of immunodeficiency that can result in the reactivation of viruses of the Herpesviridae family, and acute retinal necrosis (ARN) can occur. This article aims to evaluate the case of a patient who presented with ocular herpes reactivation during COVID-19 infection and developed bilateral ARN. A 51-year-old man presented with sudden bilateral visual loss in March 2021, 4 days after a diagnosis of COVID-19, denying any history of previous trauma or illness. He sought medical attention in his city of residence and was hospitalized for investigation ten days later. The patient was diagnosed with acute herpetic retinal necrosis, and inpatient treatment was initiated. After treatment, phacoemulsification with intraocular lens implantation and vitrectomy was performed in the left eye. In November 2021, the patient came to the Paraná Eye Hospital (Curitiba, State of Paraná, Brazil) and presented with light perception visual acuity in both eyes, right eye with phthisis bulbi, and left eye with total retinal detachment and extensive retinal fibrosis. The possibility of any intervention was ruled out for both eyes. The importance of rapid diagnosis and treatment for preserving the patient’s vision in cases of ARN can be seen. To date, only three other cases of bilateral visual loss related to COVID-19 have been reported in the literature.
Keywords: Acute retinal necrosis; COVID-19; Retinal detachment
RESUMO
A infecção por COVID-19 leva a um estado de imunodeficiência que pode resultar na reativação do vírus Herpesviridae, podendo ocorrer necrose retiniana aguda (NRA). O objetivo foi avaliar um caso de paciente que apresentou reativação herpética ocular durante infecção por COVID-19, desenvolvendo necrose retiniana aguda bilateral. Homem, 51 anos, apresentou perda visual súbita bilateral em março de 2021, quatro dias após diagnóstico de COVID-19, negando história de trauma ou doença prévia. Buscou atendimento médico em sua cidade, internado para investigação dez dias após. Diagnosticado com necrose retiniana aguda herpética, iniciou tratamento hospitalar. Após o tratamento, foi realizado, em olho esquerdo, facoemulsificação com implante de lente intraocular e vitrectomia. Em novembro de 2021, buscou o Hospital de Olhos do Paraná (Curitiba, Paraná, Brasil), apresentando acuidade visual de percepção luminosa em ambos os olhos, olho direito com phtisis bulbi e olho esquerdo com descolamento total de retina com extensa fibrose retiniana, sendo descartada, em ambos os olhos, possibilidade de intervenção. Nota-se a importância de um rápido diagnóstico e tratamento para a manutenção da visão do paciente em caso de necrose retiniana aguda. Até o momento, apenas três outros casos de perda visual bilateral envolvendo COVID-19 foram relatados na literatura.
Palavras-chave: Necrose retiniana aguda; COVID-19; Descolamento de retina
INTRODUCTION
The 2019 novel coronavirus (SARS-CoV-2) caused the recent COVID19 pandemic. Clinical presentations of the disease can range from asymptomatic or mild flu-like symptoms to severe respiratory distress. Various ocular manifestations have also been reported, and these are more common in patients with severe systemic diseases that have altered blood and inflammatory parameters1,2.
COVID19 infection is also associated with biological factors and clinical markers of acquired immunosuppression, such as lymphopenia, eosinopenia, and multiple organ failure, resulting in a dysfunctional immune response to the infectious agent3-5. This state of immunodeficiency and immune dysregulation can result in reactivation of viruses of the Herpesviridae family5,6. Acute retinal necrosis (ARN) is a bilateral granulomatous panuveitis caused by herpes viruses6,7.
The first description of ocular manifestations of COVID-19 was reported by Wu et al. in a case series of 38 patients in which ocular findings were present in approximately 31.5% of patients1. That report described the presence of multiple hyperreflective retinal lesions in the ganglion cell layer and the inner plexiform layer, as well as cotton-like lesions and microhemorrhages on retinal mapping.
To date, few reports of acute herpes virus retinal necrosis triggered by immunosuppression due to COVID19 infection have been published in the world and Brazilian literature. Thus, this report aims to provide information that can help the medical community by detailing the profile of the affected patient and the characteristics that led to the ARN clinical picture.
CASE REPORT
In March 2021, a 51-year-old brown man reported sudden bilateral visual loss 4 days after a diagnosis of COVID19, while denying any history of previous trauma or illness. The patient reported seeking emergency medical care in his city of residence one day after the onset of the condition. He was hospitalized for investigation 10 days later on April 2021.
According to the patient’s medical records at the time, in addition to symptomatic treatment for COVID19, retinal mapping was performed, showing vitreitis and associated areas of retinitis in both eyes. On ocular ultrasound, retinal detachment was observed in the left eye. Vitreous humor samples tested positive for HSV1, confirming a diagnosis of herpetic ARN. Inpatient treatment was started with 800mg intravenous (IV) aciclovir every 8 h for 14 days and 60mg oral prednisone for eleven days, followed by outpatient treatment with 2g daily oral aciclovir and 20mg daily prednisone.
Due to the antiviral treatment, the hospital service in charge of the patient chose to postpone retinal surgery until a month after the beginning of the hospital treatment, when phacoemulsification with intraocular lens (IOL) implantation and vitrectomy via pars plana (VVPP) with oil was performed in the left eye. The patient reported having had ophthalmological follow-up since the event, but denied any improvement in his vision.
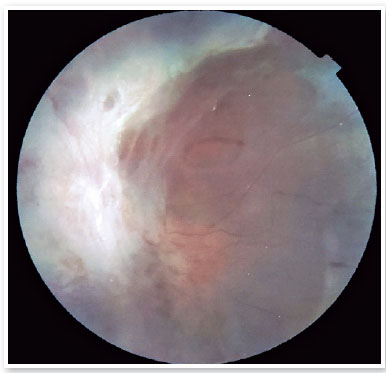
In November 2021, the patient sought care at the Paraná Eye Hospital (Curitiba, State of Paraná, Brazil) for second opinion on his condition. He had a best-corrected visual acuity of light perception (LP) in both eyes and an intraocular pressure of 10mmHg in both eyes. On biomicroscopy, a white cataract was observed in the right eye (Figure 1), and a topical IOL was found in the left eye, with a white retinal reflection under a red field. Retinal mapping could not be performed in the right eye due to opacity (white cata-ract), while the left eye had an extensive area of retinal fibrosis affecting the entire posterior pole and the middle and extreme periphery, making it impossible to evaluate the macula, the optic nerve, and other retinal structures (Figure 2).

In December 2021, the patient returned for an evaluation by the Retina Department team at the Paraná Eye Hospital, having maintained a visual acuity of light perception and showing an intraocular pressure of 6 mmHg in both eyes. Retinal mapping was impossible in the right eye due to the white cataract, and the left eye showed total retinal detachment with extensive retinal fibrosis. An ultrasound of the right eye showed phthisis bulbi with diffuse retinal thickening. Because of the advanced stage of the condition and poor visual prognosis, any intervention was ruled out for both eyes.
DISCUSSION
This is the report of a case of sudden bilateral vision loss associated with unilateral retinal detachment following herpes virus reactivation due to immunosuppression caused by COVID19 infection. To the best of our knowledge, only three other cases of bilateral visual loss due to COVID19 have been reported so far in the literature4,7.
Since the beginning of the pandemic, a few cases of post-COVID19 ARN have been described. For example, Mishra et al. reported the case of a 71-year-old male patient with unilateral involvement6, and Gonzales et al. reported a 32-year-old female patient with unilateral involvement5, while Hassanpour et al. reported a 58-year-old female patient with unilateral involvement3. However, cases with bilateral involvement after COVID19 infection are rarer, with the only cases reported so far being those by Gupta et al., of a 75-year-old male patient7, and by Soni et al. of two male patients, one 5 years old and other 61 years old4.
COVID19 infection causes mild-to-severe respiratory symptoms and several systemic manifestations. An exacerbated host response to infection is considered to cause dysregulation of the immune system itself, and a decrease in the amounts of CD3, CD4, and CD8 T lymphocytes can occur, with the potential for reactivation of viruses of the Herpesviridae family, leading to a clinical picture of ARN8,9.
ARN is considered to be a disease caused by an infectious trigger leading to severe inflammation with immune-mediated obliterative vasculitis. It is considered to be an infectious syndrome caused by members of the Herpesviridae family, affecting patients ranging from immunocompromised to immunocompetent, with no distinction by age or sex3,7-10. The main causative agent of ARN is the varicella-zoster virus, followed by herpes simplex viruses (HSV1 and 2), and less frequently cytomegalovirus (CMV) and the Epstein-Barr virus (EBV)8-10. The clinical picture of ARN patients usually includes rapidly deteriorating visual acuity, with a poor prognosis, along with vitreitis, retinitis, retinal ischemia and edema, and retinal detachment. Retinal fibrosis is possible later on, and retinal detachment is the leading cause of worsening vision in these patients7-10. A meta-analysis evaluating 67 studies and 1811 cases of ARN showed an overall incidence of 47% for retinal detachment8.
The treatment of ARN is intravenous aciclovir 10mg/kg every 8 h or 1500mg/m2 a day divided into three doses for 7-10 days, followed by oral antiviral treatment on an outpatient basis. New drugs with higher bioavailability have been shown to be effective, such as ganciclovir, valaciclovir, famciclovir, and foscarnet.5-10 Some authors advocate introducing oral corticosteroids two days after starting antiviral treatment for 10 days3,8,9. A meta-analysis by Zhao et al.8 showed that systemic antiviral therapy can reduce the rate of retinal detachment from 67% to 43%, and of prophylactic vitrectomy from 45% to 22%, both with statistical significance. When systemic antiviral therapy was associated with prophylactic vitrectomy, the lowest retinal detachment rate (18%) was observed.
In the presently reported case, there was 10 days delay from the onset of symptoms to the start of hospital treatment, as well as a wait for the end of intravenous and ambulatory antiviral therapy before performing vitrectomy on the patient. According to the literature, these delays would be significant for the unfortunate outcome of this case.
Although rare, bilateral ARN involvement involving the recent COVID19 infection is possible. Severe, rapidly progressing cases, such as the one in the present report, highlight the great importance of a quick diagnosis and treatment for the maintenance of the patient’s vision in ARN cases, since any delay in this process can cause major complications with significant impairment of the patient’s visual acuity, which can be irreversible.
REFERENCES
1. Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020; 138(5):575-578.
2. Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: a review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69(3):488-509.
3. Hassanpour K, Khorasanizadeh F, Ahmadied H, Nabavi M, Daftarian N, Ramezani A. Varicella Zoster Virus Induced Acute Retinal Necrosis Following Acute Meningoencephalitis in a Patient with Presumed COVID-19. Research Square, 2020.
4. Soni A, Narayanan R, Tyagi M, Belenje A, Basu S. Acute Retinal Necrosis as a presenting ophthalmic manifestation in COVID 19 recovered patients. Ocul Immunol Inflamm. 2021;29(4):722-725.
5. Gonzalez MP, Rios R, Pappaterra M, Hernandez M, Toledo A, Santos C, et al. Reactivation of Acute Retinal Necrosis following SARS-CoV-2 Infection. Case Rep Ophthalmol Med. 2021 Jul 16:7336488.
6. Mishra SB, Mahendradas P, Kawali A, Sanjay S, Shetty R. Reactivation of varicella zoster infection presenting as acute retinal necrosis post COVID 19 vaccination in an Asian Indian male. Eur J Ophthalmol. 2023:33(1):NP32-NP36.
7. Gupta A, Dixit B, Stamoulas K, Akshikar R. Atypical bilateral acute retinal necrosis in a coronavirus disease 2019 positive immunosuppressed patient. Eur J Ophthalmol. 2022;32(1):NP94NP96.
8. Zhao XY, Meng LH, Zhang WF, Wang DY, Chen YX. Retinal Detachment After Acute Retinal Necrosis and The Efficacies of Different Interventions: A Systematic Review and Metanalysis. Retina. 2021;41(5):965-978.
9. Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of Acute Retinal Necrosis. Ophthalmology. 2010; 117(4):818-824.
10. Powell B, Wang D, Llop S, Rosen RB. Management Strategies of Acute Retinal Necrosis: Current Perspectives. Clin Ophthalmol. 2020;8(14):1931-1943.
AUTHOR’S INFORMATION

Funding: No specific financial support was available for this study.
Conflict of interest: None of the authors have any potential conflict of interest to disclose.
Received on:
October 10, 2022.
Accepted on:
March 11, 2023.