Juliana Albano de Guimarães1; Frederico Castelo Moura2,3
DOI: 10.17545/eOftalmo/2022.0011
ABSTRACT
We present the case of a 45-year-old male patient with sudden painless visual loss in the left eye (OS), diagnosed as nonarteritic anterior ischemic optic neuropathy (NAION). Three months later, a new superior scotoma, accompanied by an inferior optic disc edema, characterized a recurrent episode of NAION in OS. Ambulatory blood pressure monitoring disclosed arterial hypertension, with no pressure drop during sleep. Optical Coherence Tomography showed optic disc drusen. Ipsilateral recurrence of NAION is a rare event. We believe insufficient relief of the “crowding” effect in predisposed optic discs after a sectoral NAION episode is an important risk factor for ipsilateral recurrences.
Keywords: Optic neuropathy; Ischemic; Optic disk; Optic disk drusen; Optic nerve diseases; Hypotension.
RESUMO
Apresentamos o caso de um paciente do sexo masculino, 45 anos, com perda visual súbita e indolor no olho esquerdo (OE), diagnosticada como neuropatia óptica isquêmica anterior não arterítica (NOIANA). Três meses depois, novo escotoma superior, acompanhado de edema inferior do disco óptico, caracterizou um episódio recorrente de NOIANA em OE. Monitorização ambulatorial da pressão arterial revelou hipertensão arterial, sem quedas de pressão durante o sono. A tomografia de coerência óptica mostrou drusas de disco óptico. A recorrência ipsilateral de NOIANA é um evento raro. Os autores acreditam que o alívio insuficiente do efeito de “aglomeração”, em discos ópticos predispostos, após um episódio setorial de NOIANA, seja um importante fator de risco para recorrências ipsilaterais.
Palavras-chave: Neuropatia óptica; isquêmica; Disco óptico, drusas no disco óptico; Doenças do nervo óptico; Hipotensão.
INTRODUCTION
Ipsilateral recurrences of nonarteritic anterior ischemic optic neuropathy (NAION) are rare events, with a reported incidence of 3.6-6.4%.(1) Many hypotheses have been set forth to explain these low rates, but the topic remains controversial1-4. We report a case of recurrent NAION and discuss the pathophysiological mechanisms of the condition.
CASE REPORT
A 45-year-old man reported waking up with painless visual loss in the left eye (OS). Clinical and ophthalmological history was unremarkable. Diabetes, hypertension, dyslipidemia and smoking were denied. Visual acuity (VA) was 20/20 in the right eye (OD) and 20/25 in OS. A relative afferent pupillary defect was evident in OS. Fundoscopy revealed a superior sectoral edema of the optic disc in OS and a crowded optic disc in OD, with a cup-to-disc ratio of 0.2 (Figure 1A). Humphrey 24.2 perimetry revealed an inferior altitudinal defect in OS (Figure 1B), supporting a tentative diagnosis of NAION. Blood pressure was 140 x 90 mmHg.
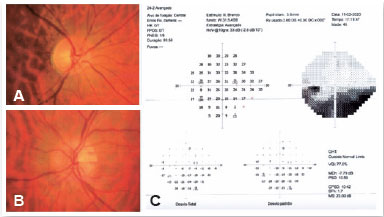
No changes were seen on magnetic resonance imaging. Blood count, C-reactive protein concentration and erythrocyte sedimentation rate were normal. Myelin oligodendrocyte glycoprotein (MOG) antibodies, aquaporin-4 (AQP4) antibodies, rheumatoid factor, antinuclear antibodies, serum angiotensin-converting enzyme, antineutrophil cytoplasmic antibody, vitamin B12 and serologies for HIV, syphilis, tuberculosis and cat-scratch disease were negative or within reference values. Despite clinical features suggestive of NAION, pulse therapy with methylprednisolone was administered upon prescription by the neurologist. No improvement in VA ensued.
Three months after the initial episode, the patient reported a new sudden visual loss (a superior extension of the scotoma) in the same eye. VA decreased to 20/60 in OS, and an inferior sectoral edema of the left optic nerve was detected on fundoscopy (Figure 2A). On perimetry, a superior paracentral scotoma was observed in OS, in addition to the previous inferior altitudinal defect (Figure 2B). The radiological and laboratory findings were normal. Oral corticosteroid therapy was instituted, but VA remained unchanged.
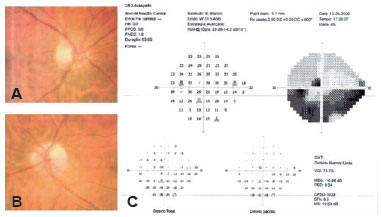
A cardiological investigation was conducted, including a 24-hour ambulatory blood pressure monitoring (73 measurements) showing a mean systolic blood pressure of 151 mmHg and diastolic blood pressure of 107 mmHg. The lowest diastolic blood pressure was 62 mmHg during sleep and 55 mmHg during wakefulness. The highest systolic blood pressure was 173 mmHg during sleep and 183 mmHg during wakefulness. Pressure did not decrease during sleep, including the patient in the “non-dipping” profile. Electrocardiography showed mild left ventricular hypertrophy. Although not obese nor snorer, obstructive sleep apnea was ruled out after polysomnography. Oral hypotensive agents were prescribed for systemic arterial hypertension. During follow-up, an optic coherence tomography (OCT) of the optic nerve was performed and showed loss of superior and inferior nerve fibers in OS, in addition to a cup-to-disc ratio of 0.2 and suggestive images of drusen. VA in OS stabilized at 20/60.
DISCUSSION
Three major theories have been proposed to explain the low incidence of ipsilateral recurrence of NAION. The first one, in fact, justifies the low clinical incidence for a lack of diagnosis, once patients would be unlikely to notice new visual loss due to the diffuse scotoma produced by the original event. This is hardly tenable since most new visual field defects are symptomatic and perceived by the patient2, as in the case reported here. The second theory posits that spared nerve fibers receive additional perfusion from capillaries of the affected portion through a shunting mechanism, thereby protecting the optic nerve from new ischemic events2, but this has been ruled out by studies evaluating the perfusion of the optic nerve head1.
The third theory invokes decompression of the optic nerve due to loss of nerve fibers secondary to NAION1,3,4. This theory is consistent with the typical crowded and cupless optic disc morphology which is believed to predispose to NAION since axons are more prone to compression and consequent ischemia when the volume in the space delimited by the inextensible lamina cribrosa and scleral opening is increased. Also supporting the hypothesis of insufficient decompression increasing the likelihood of NAION recurrence, the presence of optic nerve drusen may contribute to the maintenance of crowding, despite a previous NAION episode, and thus predispose to recurrence, as suggested by Al-Bakri et al.5.
Hayreh et al. refute the hypothesis of low incidence of ipsilateral recurrence being due to axonal decompression by arguing that optic disc cupping does not increase after an episode of NAION. Rather, discs maintain their crowded aspect due to a secondary gliotic process. According to these authors, the occurrence of new ipsilateral episodes would require the presence of other determining factors, the main one being hypoperfusion of the optic nerve head due to a significant decrease in mean diastolic pressure during slee1. We certainly agree that hypoperfusion of the optic nerve head contributes to the pathophysiology of ipsilateral recurrences of NAION, but believe crowding plays a significant role as well, mainly when associated with drusen. Although the secondary gliotic tissue that replace lost nerve fibers after a first episode of NAION will not evolve with edema, as occurs in atrophic optic discs, the remaining nerve fibers may become swollen and, in an already crowded disc, lead to ischemia.
In common with cases reported by Beck et al.2, Borchert et al.4, Hamed et al.3 and Pepple et al.6, our case initially presented with a sectoral edema of the optic disc. We believe sectoral involvement would be associated with less decompression of the optic disc nerve fibers than necessary to offset the crowding effect, increasing the likelihood of recurrence.
Another argument in favor of this hypothesis is the proportionally greater incidence of ipsilateral recurrence in young patients1. Although NAION has been reported mostly in patients over the age of 50 years, there is increasing awareness that NAION can also occur in younger patients, mainly when cardiovascular risk factors are present7. Hamann et al recently disclosed that optic disc drusen are significantly more frequent in patients with NAION younger than 50 years-old than expected in general population8, what would contribute to the proportionally greater incidence of ipsilateral recurrence in comparison with older NAION patients.
In conclusion, the ipsilateral recurrence of NAION involves a combination of multiple unusual events and factors. We believe the lesser involvement of the nerve fibers in sectoral NAION is one of the likely mechanisms, along with the presence of optic nerve drusen, which appears to contribute to crowding in predisposed optic nerves.
REFERENCES
1. Hayreh SS, Podhajsky PA, Zimmerman B. Ipsilateral recurrence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132(5):734-42.
2. Beck RW, Savino PJ, Schatz NJ, Smith CH, Sergott R. Anterior ischaemic optic neuropathy: Recurrent episodes in the same eye. Br J Ophthalmol. 1983;67(10):705-9.
3. Hamed LM, Purvin V, Rosenberg M. Recurrent anterior ischemic optic neuropathy in young adults. J Clin Neuroophthalmol. 1988; 8(4):239-46.
4. Borchert M, Lessell S. Progressive and recurrent nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1988; 106(4):443-9.
5. Al-Bakri M, Larsen AC, Malmqvist L, Hamann S. Ipsilateral Recurrence of Optic Disc Drusen-Associated Anterior Ischemic Optic Neuropathy in a 15-Year-Old Boy. J Neuroophthalmol. 2021; 41(1):e36-8.
6. Pepple KL, Bhatti MT, Foroozan R. Not again! Surv Ophthalmol. 2011;56(1):86-93.
7. Behbehani R, Ali A, Al-Moosa A. Risk factors and visual outcome of Non-Arteritic Ischemic Optic Neuropathy (NAION): Experience of a tertiary center in Kuwait. PLoS One. 2021;16(2 February):e0247126.
8. Hamann S, Malmqvist L, Wegener M, Fard MA, Biousse V, Bursztyn L, Citirak G, Costello F, Crum AV, Digre K, Fraser JA, Huna-Baro R, Katz B, Lawlor M, Newman NJ, Peragallo JH, Petzold A, Sibony PA, Subramanian PS, Warner JEA, Wong SH, Fraser CL, Optic Disc Drusen Studies Consortium. Young Adults With Anterior Ischemic Optic Neuropathy: A Multicenter Optic Disc Drusen Study. Am J Ophthalmol. 2020 Sep;217:174-81.
AUTHOR’S INFORMATION


Funding: No specific financial support was available for this study
Conflict of interest: None of the authors have any potential conflict of interest to disclose
Received on:
June 24, 2021.
Accepted on:
June 1, 2022.