Luísa Grave Gross; Renata Diniz Lemos; Vinícius Dantas Almeida; Matheus Schwengber Gasparini; Arthur Pinheiro Favarato; José Paulo Cabral de Vasconcellos; Cesar Rodrigues de Lima Neto
DOI: 10.17545/eOftalmo/2021.0020
ABSTRACT
Glaucoma is a chronic optic neuropathy that is the main cause of irreversible blindness in the world. One of the surgical methods currently available for its treatment is the implantation of the Ahmed valve, a device capable of performing a shunt between the anterior chamber and the subconjunctival region, diverting the flow of aqueous humor and, consequently, reducing intraocular pressure - the only modifiable parameter in disease progression. This work describes a case of spontaneous extrusion of the Ahmed valve implanted in the lower nasal region. It is a rare complication of this type of implant, which occurred on the 60th postoperative day, not triggered by eye trauma or other factors. The number of previous eye surgeries is a condition that may have contributed to the occurrence of extrusion. The identification and appropriate treatment of such complications help to reduce possible infections secondary to extrusion, such as endophthalmitis.
Keywords: Ahmed glaucoma valve; Refractory glaucoma; Spontaneous extrusion.
RESUMO
O glaucoma é uma neuropatia óptica crônica que figura como a principal causa de cegueira irreversível no mundo. Um dos métodos cirúrgicos disponíveis atualmente para o seu tratamento é o implante da válvula de Ahmed, dispositivo capaz de realizar um shunt entre a câmara anterior e a região subconjuntival, desviando o fluxo do humor aquoso e, consequentemente, gerando redução da pressão intraocular – o único parâmetro modificável na progressão da doença. Este trabalho descreve um caso de extrusão espontânea da válvula de Ahmed implantada em região nasal inferior. Trata-se de uma complicação rara deste tipo de implante, ocorrido no 60º dia de pós-operatório, não desencadeada por trauma ocular ou outros fatores. O número de cirurgias oculares prévias é uma condição que pode ter contribuído para a ocorrência da extrusão. A identificação e o tratamento adequado de tais complicações auxiliam na redução de possíveis infecções secundárias à extrusão, como a endoftalmite.
Palavras-chave: Válvula de Ahmed; Glaucoma avançado; Extrusão espontânea.
INTRODUCTION
Glaucoma is a chronic optic neuropathy that is the leading cause of irreversible blindness in the world1. Its pathophysiology involves changes in the neuroretinal rim of the optic disc and progressive loss of retinal ganglion cells, causing constriction of the visual field2.
In 2010, 60.5 million individuals were estimated to be affected by primary open-angle glaucoma or primary closed-angle glaucoma worldwide. Tham et al. determined the overall incidence of glaucoma to be 3.54%, with Africa being the most affected continent. By 2040, this number is expected to increase to 111.8 million individuals, with most of the patients from Asia and Africa1.
Several risk factors are associated with the development and progression of glaucoma; some of them are age, family history, black race, and myopia2. Reduction in the intraocular pressure (IOP) is the only modifiable parameter in the natural history of glaucoma. The effect of reduction in IOP on slowing the progression of glaucoma is already established. IOP can be reduced using eye hypotensive medications, laser therapy, or surgery3.
One of the surgical methods is the implantation of an Ahmed valve, a device capable of acting as a shunt between the anterior chamber (AC) and subconjunctival region, diverting the aqueous humor from the AC to a posterior external reservoir from where it is resorbed, thus causing a reduction in IOP4. The use of Ahmed valves is increasing, and many consider it to be the first-choice surgery for treating glaucoma5.
In this study, we aimed to describe a case of spontaneous extrusion of the Ahmed valve, a rare complication of this implant type, and to highlight the relevant aspects already published in the literature regarding the possible complications related to the treatment of advanced glaucoma using this device.
CASE REPORT
A 73-year-old female patient diagnosed with primary open-angle glaucoma in both the eyes (OU) 8 years ago underwent trabeculectomy surgery with mitomycin C in OU in 2017 due to the progression of glaucoma despite regular use of four types of hypotensive eye drops. During the postoperative follow-up of the right eye (OD), a progressive increase in IOP due to subconjunctival healing of a filtering bleb was observed, and the patient received topical injections of mitomycin C twice without success. Nine months after the first surgery, trabeculectomy was performed in OD.
In 2019, the new filtering bleb healed and consequently IOP failed to be controlled. Therefore, Ahmed valve implantation in the upper temporal region was indicated. One month postoperatively, the implant had to be removed due to ocular hypotony with grade III athalamy, refractory to several attempts to fill the AC with methylcellulose and tube suture.
Three months later, the patient was using four classes of hypotensive eye drops in OD, in addition to acetazolamide 250 mg orally four times a day; however, IOP remained around 17 mmHg. The patient had a visual acuity (VA) of 0.3 in OD and 0.9 × 0.9 cupping in the same eye. Thereafter, another Ahmed valve implantation was performed in the lower nasal region that required a conjunctival graft from the contralateral eye to properly cover the implant as well as the scleral patch.
During the first 15 days postoperatively, IOP remained around 10 mmHg. On postoperative day 30, a fixed combination of timolol 0.5% and brimonidine 0.1% was administered due to an IOP of 16 mmHg. On postoperative day 60, the patient sought the ophthalmological emergency room of the State University of Campinas Clinics Hospital with complete extrusion of the valve (Figure 1). The patient denied any type of trauma, eye pruritus, or other possible triggering factors.
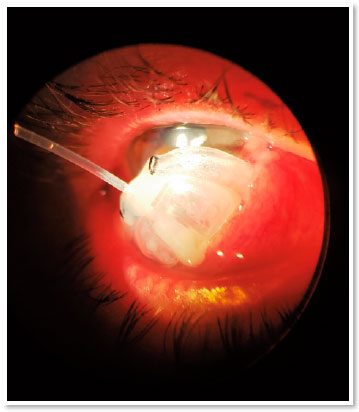
After this complication, four classes of hypotensive eye drops and acetazolamide 125 mg orally were reintroduced three times a day (due to intolerance to higher doses), thus controlling IOP at 10 mmHg. The patient maintained a VA of 0.3 OD, 0.9 × 0.9 cupping and no evidence of choroidal detachment; the patient continues to be followed up.
DISCUSSION
The Ahmed valve comprises a plate connected to a silicone tube, with external and internal diameters of 600 and 300 μm, respectively. For its implantation, the tube is curved at the limbus in the direction of the AC of the eyeball, usually at an angle of 90°. The plate is later sutured to the sclera4. This device diverts the aqueous humor from the AC to a posterior external reservoir where it is resorbed, thereby reducing IOP. Its use is generally limited to patients with refractory glaucoma or who have unsuccessfully undergone other surgeries for glaucoma.
Some complications related to the Ahmed valve include ocular hypotony, corneal decompensation due to the tube touching the endothelium, conjunctival dehiscence, excess fibrosis around the plate, implant exposure, and other rare infections5. Extrusion through the conjunctiva and contact of the tube with the endothelium are tube-related complications. Exposure of any part of the valve, i.e., the tube or plate, is considered as an eye emergency because it can cause endophthalmitis due to the migration of microorganisms from the eye surface and conjunctiva into the eyeball4.
Spontaneous extrusion of this device is extremely rare, and only two cases have been reported in MEDLINE®. In the first case, the extrusion occurred 3 months after the successful treatment of postoperative orbital cellulitis6. In the second case, Ahmed valve implantation was performed to correct drug-refractory glaucoma after eye trauma. The latter case report does not specify how long after the procedure the complication occurred.7 In addition, there is a report on the imminent extrusion of another type of drainage implant, known as the Ex-PRESS8.
Several studies have shown that the site of device implantation affects the risk of tube exposure, with the inferior region having the highest risk5,9. This is believed to occur due to greater exposure of both the conjunctiva and anterior portion of the device, in addition to the mechanical trauma caused by the lower eyelid. Therefore, many surgeons prefer the upper temporal region for Ahmed valve implantation because, in this region, the valve is covered by the upper eyelid. Other factors that contribute to conjunctival damage and consequent exposure of the tube are the use of multiple hypotensive eye drops, previous eye surgeries, and eye trauma9,10.
Byun et al. studied factors such as age, type of glaucoma, diabetes mellitus, and systemic arterial hypertension to identify whether these conditions had a significant relationship with partial exposure of the device. In that study, none of these characteristics significantly affected the exposure of the implant; the number of previous eye surgical procedures was the only significant risk factor11.
Several techniques such as the rotation of the scleral flap and advancement and duplication of the Tenon capsule have been proposed to cover the tube after extrusion. Bovine pericardium, dura mater, amniotic membrane, and sclera are some of the materials used for this purpose4. However, in many cases, these techniques have proven to be inefficient in preventing re-exposure of the tube4.
Recently, Nardi et al. proposed a new surgical technique to manage the exposure of the Ahmed valve tube. They argued that the previous techniques failed due to the effort to cover the exposure without correcting its cause, which consequently would more easily lead to a re-exposure. According to this theory, as the tube is made of an elastic material, when the tube is bent for entry into the AC, it will tend to return to its original position. Only a rigid entry site would prevent this. If the entry site is weakened, the tube may return to its original position, and head toward the corneal endothelium4. Further, they stated that two important advantages may result from using a greater radius of curvature when introducing the tube into the AC. First, with a longer intrascleral trajectory, the position of the tube in the AC would become more stable, and second, the possible force against the valve cover material as well as against the conjunctiva would be lower, thereby reducing the force vector perpendicular to the conjunctiva4. Subsequently, they added an entry point into the AC more obliquely than the valve axis and used a scleral patch with fibrin glue, thus providing a waterproof closure around the entry point of the tube. This results in a deep AC and without inflammation on postoperative day 1, concomitant with the start of tube function4.
In the present case, the patient had undergone several surgeries in the right eyeball before undergoing Ahmed valve implantation in the lower nasal region with a conjunctival graft in the same eye, leading to areas of fibrosis in both the conjunctiva and sclera. This makes a possible new ocular surgery technically more difficult and complex as well as increases the postoperative risks of complications due to increased inflammation and difficulty in healing. In addition, the chronic use of hypotensive eye drops may have contributed to conjunctival erosion and may be associated with the occurrence of spontaneous extrusion10.
In conclusion, we discussed a rare complication related to Ahmed valve implantation. In the vast majority of cases, it is effective in reducing IOP and is therefore indicated to control the progression of glaucoma in advanced stages or refractory to other treatments. Ophthalmologists should keep in mind the possible complications resulting from this procedure to identify and treat them properly; they should also identify the patients who have the most risk factors for failure of device implantation.
REFERENCES
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90.
2. McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71-8.
3. Rulli E, Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573-82.
4. Nardi M, Maglionico MN, Posarelli C, Figus M. Managing Ahmed Glaucoma Valve tube exposure: Surgical technique. Eur J Ophthalmol. 2020:1120672120925641.
5. Riva I, Roberti G, Oddone F, Konstas AG, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357-67.
6. AlDarrab A, AlBahlal A, Dibaji M, AlZaid A, AlJadaan I, Elkhamary S, et al. Spontaneous glaucoma drainage device extrusion after early postoperative orbital cellulitis - Case report and literature review. Saudi J Ophthalmol. 2019;33(2):192-5.
7. Yadgarov A, Liu D, Crane ES, Khouri AS. Surgical Outcomes of Ahmed or Baerveldt Tube Shunt Implantation for medically Uncontrolled Traumatic Glaucoma. J Curr Glaucoma Pract. 2017;11(1):16-21.
8. Song YJ, Kim S, Yoon GJ. Impending extrusion of Ex-PRESS shunt treated by shunt-position adjustment: a case report. BMC Ophthalmol. 2018;18(1):4.
9. Geffen N, Buys YM, Smith M, Anraku A, Alasbali T, Rachmiel R, et al. Conjunctival complications related to Ahmed glaucoma valve insertion. J Glaucoma. 2014;23(2):109-14.
10. Levinson JD, Giangiacomo AL, Beck AD, Pruett PB, Superak HM, Lynn MJ, et al. Glaucoma drainage devices: risk of exposure and infection. Am J Ophthalmol. 2015;160(3):516-21.e2.
11. Byun YS, Lee NY, Park CK. Risk factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantation. Jpn J Ophthalmol. 2009;53(2):114-9.
AUTHOR’S INFORMATION





Funding: No specific financial support was available for this study
Conflict of interest: None of the authors have any potential conflict of interest to disclose
Received on:
September 29, 2020.
Accepted on:
January 16, 2021.